Did the Lamont Glass Company (Trenton, nS, ca. 1890-1897) Produce Pressed Glass Tableware?
Conflicting Evidence from Artifacts and Folklore
J. Victor OwenSaint Mary's University
Stephen T. Powell
Nova Scotia Museum in Halifax
Résumé
De grandes quantités de vaisselle de verre sodocalcique pressé, verres de lampes à pétrole, bouteilles et autres objets de verre, ont été produites à Trenton, où trois verreries (Nova Scotia Glass Co., Lamont Glass Co. et Humphreys Glass Co.) étaient en activité entre 1881 environ et 1917. La plupart des objets de verre de Trenton étaient traditionnellement attribués à la Nova Scotia Glass Co., mais, en nous fondant sur des entrevues remontant aux années 1950 d’individus qui n’étaient pas directement liés à l’industrie, on s’aperçoit que les ouvrages de référence typologique sur le verre historique au Canada ont attribué des séries spécifiques à Lamont, en dépit du fait que quasiment aucun des objets de verre, hormis les bouteilles, ne porte de marque de fabrique. Les bouteilles incolores Lamont contiennent de 1,2 à 2,6 % d’oxyde de baryum (BaO). Cet additif accroît l’indice de réfraction du verre, lui donnant un lustre et un brillant comparable à celui du cristal de plomb, attribut nettement avantageux dans le cas du verre pressé. On n’a pas, cependant, décelé de barium dans la verrerie usuelle en Nouvelle-Écosse, ce qui permet de mettre en doute le fait que Lamont aurait produit de la verrerie de table, mais aussi la méthodologie de recherche qui a donné naissance en premier lieu à ce qui semble être un mythe.Abstract
Large amounts of soda-lime pressed glass tableware, lamp chimneys, bottles and other glass objects were manufactured in Trenton, where three glassworks (Nova Scotia Glass Co., Lamont Glass Co. and Humphreys Glass Co.) operated between ca. 1881 and 1917. Most Trenton pressed glass tableware has traditionally been assigned to the Nova Scotia Glass Co., but based on interviews dating to the 1950s of individuals not directly connected with the industry, standard reference books on historical Canadian glass have attributed specific patterns to Lamont, notwithstanding that virtually none of this glass, other than bottles, is marked by the maker. Colourless Lamont bottles contain 1.2-2.6 wt% barium oxide (BaO). This ingredient increases the refractive index of glass to which it is added, giving it a brilliant luster akin to that of lead crystal, an attribute that clearly would be advantageous in pressed glass. Barium, however, was not detected in any analyzed Nova Scotian pattern glass, thereby casting doubt not only on the suggestion that Lamont produced tableware, but also on the research methodology that gave rise to this apparent myth in the first place.1 The phrase “Nova Scotia glass” is virtually synonymous with pressed glass tableware, but large numbers of lamp chimneys, bottles and other glass objects were manufactured on Glass St. in Trenton, Nova Scotia (Fig. 1), where three neighbouring glassworks (the Nova Scotia Glass Co. (NSGCo), ca. 1881-1892, Lamont Glass Co. (LGCo), ca. 1890-1897 and Humphreys Glass Co.—on Main St., ca. 1890-1900 (?) and on Glass St., ca. 1901 (?)-1917)—competed in the late 19th century. There is considerable disagreement in the older literature on the dates of operation of these glassworks. Those reported here are from King (1987).
2 The production of pressed glass tableware has traditionally been associated with NSGCo, but based on the recollection of elderly residents in the Trenton area, early researchers (Pierce 1958; Stevens 1967) speculated that all three glassworks manufactured this type of tableware. King (1987) was unequivocal about Lamont in this regard, stating that this glassworks “made all types of glassware—bottles, tableware and industrial ware” (72)—but he also cautionedthat, as a general guide … the majority of the tableware and lamp shards [recovered from Glass St.] must have been made by the Nova Scotia Glass Co., whereas the bottle and chimney shards, unless otherwise identified, could have been made at either the Humphyres Glass Co. or Lamont Glass Co. (77)Some specific patterns of pressed glass have been attributed to either Lamont or Humphreys. Stevens (1982: 218-21), for example, attributed the “Nova Scotia Crown” (Fig. 2A; known elsewhere, including in Stevens 1982: 218, as “Pillar”) pattern to Lamont. Similarly, Pierce (1958) considered a thin-walled “Lord’s Prayer”-etched tumbler to be an “authenticated” product of the Lamont factory, evidently on the basis of the testimony of its former owner in whose family this artifact had resided for some sixty years. Stevens (1979) reported that Lorne Pierce attributed the so-called “Tassel and Crest” pattern to the Humphreys glassworks. Since this Humphreys attribution was shared by both John W. Fraser, who as a child had worked as a “carrying-in” boy at NSGCo and its successor, the Diamond Glass Co., and George MacLaren, former curator at the Nova Scotia Museum in Halifax, Stevens “feels that the term ‘authenticated’ should be applied” (1979: 152). Crown and Tassel-and-Crest pattern tableware and Lord’s Prayer tumblers may well have been made by NSGCo’s Trenton competitors, but we consider these attributions to have been based more on hearsay and folklore than convincing argument or documentation.
3 Apart from Lamont bottles bearing an em-bossed LGCo NS (or LGCo NG, the “NG” denoting the adjacent town of New Glasgow [Glass St. is within metres of the Trenton/New Glasgow town boundary]), on the base of bottles, or “LG” on the side of fruit jars, little Trenton glass was marked. For example, there are only two known marked Humphreys bottles (Bob Cunningham, pers. comm. 2004), and marked NSGCo glass appears to be confined to containers such as preserve jars. These are embossed with “Nova Scotia Glass Co. Limited, New Glasgow, N.S.—Diamond Flint grade mark” with the initials “N.S.G. Co.” enclosed by an embossed diamond (MacLaren 1971: 26). E. MacArthur (pers. comm. 2004) suggests that the reference to both the NSGCo and Diamond in this logo may indicate that these jars date to the period 1890-1892, when the Diamond Glass Co. took control of NSGCo but still operated in Trenton (see below).
4 Lamp chimneys and bottles were produced by all three glassworks. Unless marked, however, there is no straightforward, visual way of assigning any of the latter wares to a particular glassworks. However, comparison of the chemical composition of marked pieces with unidentified samples of the same type of glass provides a potential means of assigning particular objects to specific glassworks. Attention here is focused on comparing the composition of marked, colourless Lamont bottles with sherds of pressed glass tableware recovered from Glass St. The results call into question the long-held inference that some of this tableware was made by LGCo.
Historical Background
5 Glass manufacturers were attracted to the Trenton area by the abundant supply of local coal, which cost ten cents a ton in those days (Barclay 1987: 4). Moreover, the Glass St. factories were strategically located adjacent to the Intercontinental Railway, a stone’s throw from the East River (Fig. 1). W. G. Beach, NSGCo’s manager, revealed in an 1885 interview that fine, white sand used for pressed glass came from Boston (King 1987: 74-77). He was probably referring to glass-grade sand and quartzite (Cheshire quartzite) from the Berkshire Hills in western Massachusetts, from whence it was sent by rail to Boston and then exported. Indeed, the Monetary Times for October 28, 1881, reported “[t]he sand used is brought from Boston, and is known as the Berkshire sand; the lime from St. John; the soda from England and the nitrate from South America” (vol. 15, no. 18: 521). It has also been hypothesized that there were local deposits of clean silica-rich sand (Barclay 1987: 4), but this remains to be confirmed. Silica sand of Cretaceous age is known to occur in isolated areas near Shubenacadie and Musquodoboit (Fig. 1), and also in Antigonish County, so the possibility that some of this sand was used to make glass in Trenton cannot be discounted. The use of local sand by the Trenton glass industry potentially could be confirmed or refuted using trace element data (cf. Owen et al. 2005; Owen and Greenough 2008), but available information, specifically Y and Zr (Owen: unpublished) shows that the composition of at least some Nova Scotia pressed glass is consistent with the inclusion in their batches of silica sand rendered from Cheshire quartzite.
Fig. 1. Sketch map showing the location of Trenton and Moncton. Cretaceous silica sand deposits occur near Antigonish (A), Shubenacadie (S) and Musquodoboit (M). The inset shows the location of Humphreys’ Main St. operation (1) relative to the site of the Glass St. factories (2), Trenton.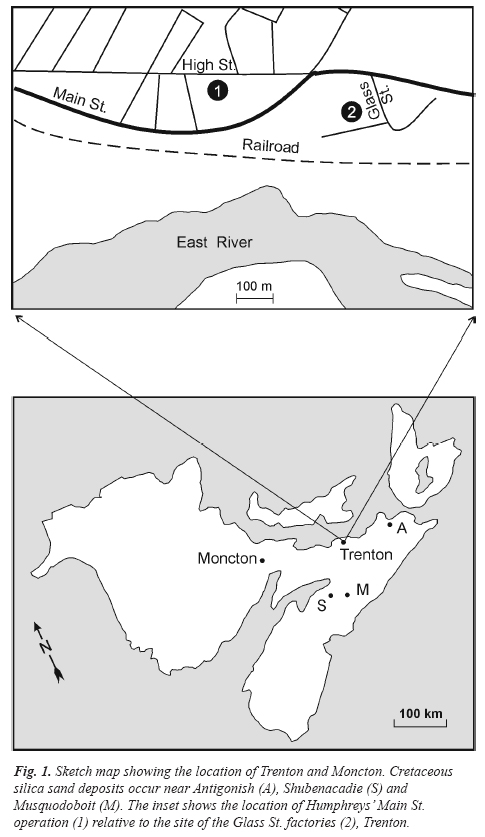
Display large image of Figure 1
6 The Nova Scotia Glass Co. initially made lamp chimneys (upwards of 9,000 per day), tableware, lanterns and globes, but by 1887, chimney production was scaled back in favour of pattern glass (MacLaren 1971). NSGCo was acquired by Diamond Glass Co. in March 1890, and subsequently by the Dominion Glass Co., but the glassworks continued its Trenton operation until November 1892, when the factory closed and its assets (notably moulds) sent to Montreal.
7 The Humphreys Glass Co. (ca. 1890-1920) commenced operations in the backyard of John Humphreys’ residence on Main St. (backing on High St.). There is considerable discrepancy in the literature about how long it operated there. According to MacLaren (1971: 19) and Wereley (2003b: 12), Humphreys moved adjacent to the NSGCo site on Glass St. later the same year (1890), but Stevens (1979: 147), citing unreferenced reports or communications with Lorne Pierce and George MacLaren, maintained that the Main St. works remained in operation until it burned ca. 1900 (?), whereupon it moved to Glass St. in ca. 1901. King suggested that the Main St. operation was experimental, and implied that in 1890 their “first” (presumably commercial) works was constructed next to the NSGCo (1987: 79). According to King, it burned in 1902, but a new glassworks was soon built at the end of Glass St. Even if Humphreys’ “experimental” operation on Main St. existed for only a few months in 1890, there still remains a large amount of glass on the site. Moreover, excavation of a water main on the site a few years ago unearthed a large number of bottles. Unfortunately, these were quickly scavenged by workmen and collectors, but their discovery at this location calls into question the hypothesis that the Main St. works was only experimental and short-lived.
8 Humphreys specialized in bottles, supplying huge numbers (as did Lamont) to Minard’s Liniment Co. as well as to various Halifax and Quebec retailers. By 1911, it was the only glassworks in Canada that operated outside the glass combine.
9 Owing to the rising cost of coal, the Humphreys works closed its Trenton operation in 1917, and moved to Moncton, New Brunswick, where cheaper, natural gas was available. The May 25, 1917 issue of the Moncton Transcript noted that in addition to making bottles, the Humphreys Moncton operation also produced lamp chimneys of all kinds, as well as undisclosed blown glass goods. By mid-July, the new factory had burned, but reconstruction began by early August; it reopened about six weeks later. In 1919, the factory workers celebrated Labour Day by holding a parade during which they carried glass canes. In June of the following year, the factory was sold to a Montreal-based mattress manufacturer.
10 The Lamont Glass Co. was established in 1890 by Donald, Henry and David Lamont. Throughout most of the 1880s, Donald Lamont had worked as a glassblower at the NSGCo, but by ca. 1890, he and his brothers started conducting experiments at producing their own glass on their farm on Little Egypt Road, about three kilometres from Glass St., Trenton. Their original intent was to produce coloured glassware (King 1987), presumably to corner a niche market not targeted by Donald Lamont’s former employer. Soon thereafter, Donald and David Lamont acquired a lot adjacent to the Nova Scotia Glass Co. on Glass St. The LGCo produced large amounts of bottles, filling orders of 300,000 for the Minard’s Liniment plant in Yarmouth, Nova Scotia, alone.
11 In April 1898, the Lamont factory was leased to the Diamond Glass Co. On August 25 of the following year, the old NSGCo buildings used by Lamont/Diamond burned. Surprisingly little was made of this event in the local media. The Pictou Advocate, for example, relegated the story to a short paragraph on page five, but among the destroyed buildings was one that was filled with Lamont/Diamond stock. This catastrophic financial loss ensured the demise of the glassworks. Donald Lamont moved to Montreal, where he continued to work for the Diamond Glass Co., but he later worked in the glass trade in central and western Canada. By the end of his career in the 1930s, he had held managerial positions in seven different glassworks (King 1987).
Sample Descriptions
12 Fourteen intact glass objects, forty-four glass sherds and nineteen pieces of cullet (broken glass used to expedite melting of glass batches) from the Glass St. site were analyzed by electron microprobe. Analytical methods have been described elsewhere (Owen 2004).
Intact Objects
13 The intact objects analyzed (Table 1) include seven marked Lamont bottles (three green, four colourless), a Panelled Jewel pattern goblet (Fig. 2A), a cover/lid of the same pattern, a Ribbed Band pattern creamer with a simple, engraved floral motif (Fig. 2B), two blown whimsy canes and two very thin, etched tumblers.
Fig. 2 Typical glass objects commonly attributed to the Trenton glassworks.
Display large image of Figure 2

Display large image of Figure 3
14 The three green Lamont bottles were made for Minard’s Liniment Co. The four colourless glass bottles lack any such logo, but six of the seven bottles have LGCo NS on their base. One colourless bottle is marked “LGCo NG” instead. One of the whimsy canes was passed down through the family of a sister of Donald Lamont. The other cane is unprovenanced but, as will be seen, it has a composition that closely resembles that of the analyzed Panelled Jewel objects. The Ribbed Band pattern pressed glass tableware produced in Trenton has a type of ribbing that apparently differs from American counterparts. It has been attributed to the Lamont factory (Pierce 1958), but the justification for this attribution was not explained.
Fig. 2B Ribbed band creamer with engraved floral motif (13.5 cm), thin walled “Lord’s Prayer” and “Home Sweet Home” tumblers.
Display large image of Figure 4
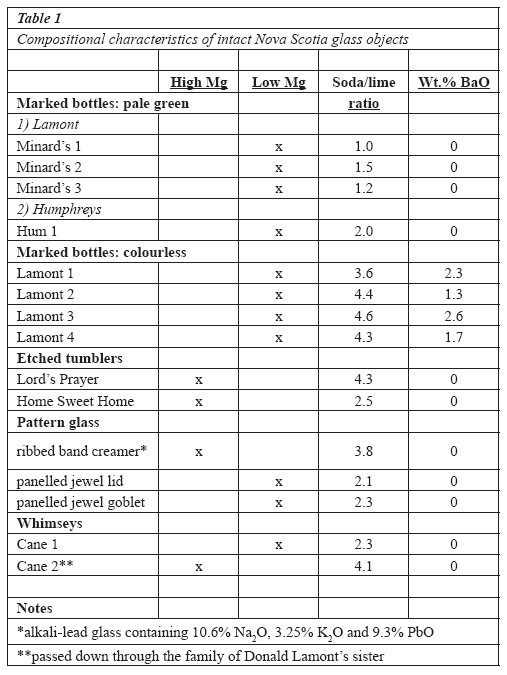
Table 1 Compositional characteristics of intact Nova Scotia glass objects
Display large image of Table 1
15 One of the tumblers is etched with the “Lord’s Prayer”; the other has a “Home Sweet Home” poem (Fig. 2B). These types of tumblers have traditionally been assigned to the Lamont factory (Spence 1966: 59; Stevens 1979: 157; Unitt 1969: 20).
Glass St. artifacts
16 The analyzed Glass St. site comprises material excavated in the 1960s by George MacLaren of the Nova Scotia Museum (McLaren 1971), and subsequently by “Glassfax” members. It consists of thirty-three fragments of pressed glass representing a total of eighteen distinct patterns (Table 2); four pieces of suspected waste glass—including rough glass adhered to sand—and glass blobs and threads (Table 3), and seven malformed (distorted), shaped objects (i.e., wasters) including pattern glass objects and lamp chimneys (Table 3). In addition, nineteen pieces of cullet spanning a wide range of colours were analyzed (Table 4). We emphasize that although our characterization of particular samples as being waste glass is in keeping with the traditional interpretation of similar material elsewhere, it can be difficult to prove that the glass preserved as rinds on raw materials (e.g., quartz sand or sandstone), kiln artifacts and the like, was deliberately made at the site where it was found (Owen and Culhane 2005). However, the composition of this material matches—or at least overlaps—that of some of the wasters and marked bottles analyzed here, so the traditional interpretation would seem to be valid in this instance.
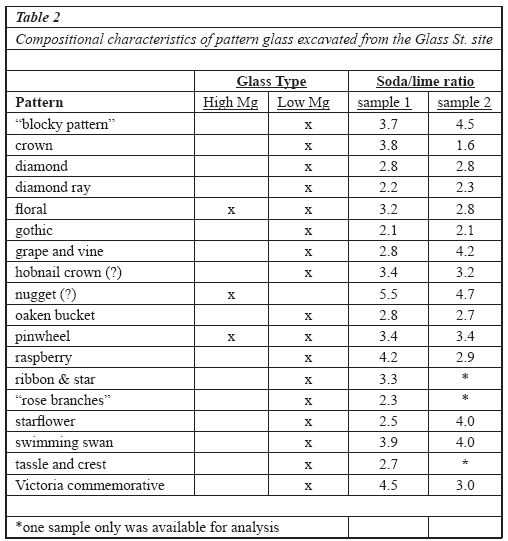
Table 2 Compositional characteristics of pattern glass excavated from the Glass St. site
Analytical Results
17 Apart from four specimens with alkali-lead (Na-K-Pb) compositions (three cullet samples and the Ribbed Band creamer), all of the artifacts described here consist of soda-lime glass. Most have low magnesia contents (MgO<0.5 wt.%), but some are enriched (1-5% MgO) in this component. It probably originates in dolomite or dolomitic limestone added (along with limestone) as a stabilizer in glass batches. Silica (SiO2) contents of the soda-lime glass samples range from 68 to 78 per cent. In the interest of brevity, only key aspects of the analytical database established for these samples are reported here.
Intact Objects
18 The marked bottles all have low-Mg compositions. Their soda/lime (Na2O/CaO) ratios vary according to the colour of the glass. The pale Humphreys and Lamont bottles have soda/lime ratios about half or less (1.0-2.0 vs. 3.6-4.6) that of the colourless Lamont bottles (Table 1). Most significantly, barium was detected only in the latter four samples (Table 1).
19 Three intact specimens of pattern glass (Ribbed Band and Panelled Jewel) were analyzed. The two panelled jewel objects (a goblet and a lid or cover for a bowl) have very similar compositions, with soda/lime ratios of about 2.2, but the ribbed band creamer is a completely different type of glass (i.e., a magnesian, alkali-lead glass, rather than a low-Mg, soda-lime glass). Alkali-lead glass cullet was recovered from the Glass St. site, but unlike the creamer, this cullet is strongly coloured (Table 4). It is noteworthy that the Panelled Jewel goblet is identical to one illustrated in an 1886 NSGCo advertisement, as reproduced by Barclay (1987).
20 Two blown glass whimsy canes reputedly made in Trenton were also analyzed. They differ in their Mg contents and soda/lime ratios. The low-Mg glass cane has a composition very similar to the intact, panelled jewel artifacts (e.g., Na2O/CaO = 2.3; Table 1).
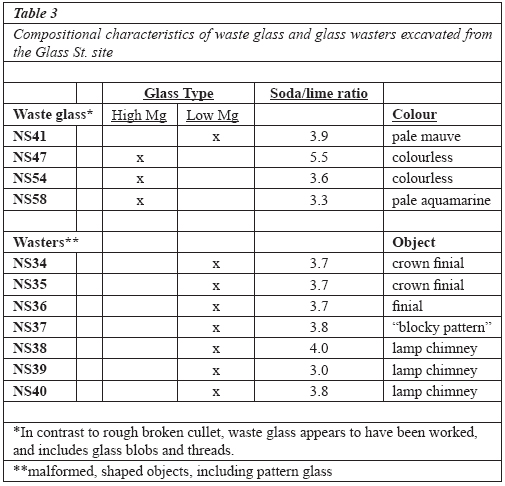
Table 3 Compositional characteristics of waste glass and glass wasters excavated from the Glass St. site
Display large image of Table 3
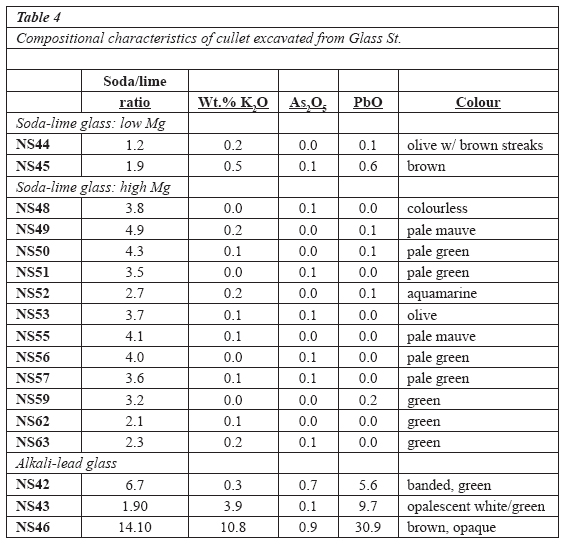
Table 4 Compositional characteristics of cullet excavated from Glass St.
Display large image of Table 4
21 The two thin-bodied tumblers with etched poems and decoration traditionally associated with Trenton glass—reputedly Lamont (Pierce 1958; Unitt and Unitt 1969)—consist of magnesian soda-lime glass (2.7%, 4.8% MgO), but they differ in their silica contents and soda/lime ratios (4.3 versus 2.5).
22 The majority of pattern glass sherds from Glass St. have very low magnesium contents (generally <0.1% MgO). Three pressed glass patterns (Floral, what tentatively has been described as Nugget and Pinwheel) can have high-Mg compositions (2.5 and 3.4% MgO, respectively). One Floral sherd, however, has a low-Mg composition. Apart from their contrasting MgO contents, the compositions of both groupings of sherds are very similar. Soda/lime ratios, however, can vary considerably within each grouping (Table 2). None contain barium above analytical detection limits.
23 Analyzed colourless waste glass and wasters from the Glass St. site have compositions (Table 3) that overlap those of the undeformed pattern glass sherds (Table 2). Both high- and low-Mg groupings are represented. Soda/lime ratios vary between 3.3 and 5.5. The compositions of wasters with recognizable patterns can closely match those of undeformed sherds of the same pattern recovered from Glass St. None contain barium above analytical detection limits.
24 Cullet compositions (Table 4) are as diverse as their colour. They comprise soda-lime (Na-Ca) and alkali-lead (KNa-Pb) glasses. Like some of the pattern glass, the soda-lime grouping includes both high-Mg and low-Mg wares. Usually used as a decolourant, all have low arsenic content—a component found in high concentrations (approaching 1% As2O5) in some of the alkali-lead cullet samples. The low-Mg soda-lime glass is distinctly calcic (Na2O/CaO = 1.2, 1.9 in two olive/brown samples) compared with its more magnesian counterpart (Na2O/CaO = 2.1-4.9 [14 samples]). None of the cullet contains barium above analytical detection limits.
Discussion
25 Enthusiasts investigating the history of the nascent Canadian glass industry often interviewed residents and owners of intact artifacts related to the local glassworks. In rare instances, the interviewee had worked in the factory of interest, so their testimony can be considered relatively reliable. An example would be Gerald Steven’s interview (Stevens 1979) of George Gardiner, who had worked as a glassblower at the Burlington Glass Works (ca. 1875-1909) in Hamilton, although the degree to which Mr. Gardiner could, late in life, “authenticate” (Stevens 1967: 80) free-blown artifacts (canes, doorstops) of the type made piecemeal at literally dozens of other 19th-century North American glassworks is dubious. Stevens, however, also endorses information provided by descendants of early glassblowers, although he cautions that “…such a source should be approached with great care and some degree of pre-knowledge” (Stevens 1967: 59). Should testimony from these well-meaning individuals be considered evidence, or folklore? Much of this testimony was eventually published in pamphlets (e.g., Pierce 1958) and standard reference books on historical Canadian glass (e.g., Stevens 1967, 1979), and thus has had a profound influence on the public’s perception of this industry and its products.
26 The appearance of these publications led to a new-found interest in the domestic glass industry in the years leading up to Canada’s centenary in 1967. Among aficionados of Canadian glass artifacts, the tableware produced in Trenton has since acquired a cachet seemingly unrivaled by the pressed glass products of any contemporary glassworks. Consequently, Trenton glass has received considerable attention in standard reference books on historical Canadian glass. Owing to fragmentary documentary records and the interview-based methodology employed by amateur historians, however, there nonetheless remains much confusion concerning the products of each of these factories. In some instances, their activities extended well beyond interviewing the elderly, and perusing archival data. Some early enthusiasts excavated the sites of historical glassworks. It is unclear whether any of this work was supervised by professional archaeologists, or if these workers themselves had any significant archaeological training. The evidence often suggests not. In many instances where glass sherds wound up in museum collections, the artifacts can lack specific sample numbers or any labelling at all, so their archaeological context is completely unknown. The same holds true of potworks sites that have been dug over by students of historical ceramics. What is clear is that the damage caused to archaeological sites by unqualified workers is permanent.
27 Contemporary documentation about historical industries is often scant, but factory advertisements, invoices and letterhead can provide an indication of the production lines of particular factories. Some of the advertisements placed by the Glass St. factories have been reproduced in the literature (King 1987; MacLaren 1971; Wereley 2003a, b). In the 1890s, for example, Lamont advertised “fruit jars and bottles of all kinds … all kinds of lamp chimneys and globes … prescriptions bottle … telegraph and telephone insulators, and battery jars.” Some of their ads also mentioned that lead glass was one of their specialties, although the kinds of objects they made from this type of glass are not specified. Humphreys advertised “all kinds of bottles, lamp chimneys, fruit jars, etc.” (Wereley 2003a: 5; 2003b: 11). Again, no mention is made of tableware.
28 To the best of our knowledge, only the NSGCo specifically mentioned tableware in the advertisements that have been reproduced in the literature. They indicate that this factory produced “Diamond flint glass, tableware, lamps, lamp chimneys, &c.” (Wereley 2003b: 15). Significantly, an advertisement published in 1886 illustrates three recognizable patterns (Panelled Jewel, Raspberry and Raspberry and Shield), as well as the engraved or etched design on another dish (Barclay 1987: 3).
29 The “etc.” (or “&c.”) mentioned in these advertisements can, of course, refer to any type of glassware, and this will continue to provoke much speculation. As it stands, however, there is no substantive evidence that either Lamont or Humphreys produced pressed, soda-lime pattern glass tableware. Lamont and Humphreys’ ads do mention that “Particular Attention [is] Given to Private Moulds” (Wereley 2003b: 11). Stevens (1979: 147) suggested that the reference to “private moulds” indicated the production of pressed pattern glass. It is more likely, however, that “private moulds” refers to the use of replaceable engraved metal plates that could be fitted into bottle moulds to customize these containers according to the requirements of clients (King 1987: 78).
30 It is clear that the historical and archival record of the glass-making industry in Trenton is incomplete, and in places, ambiguous. Analytical data, however, can provide additional clues about who produced what. Eighteen patterns of pressed glass from Glass St. were analyzed, more than half of all patterns known to have been produced in Nova Scotia. None of these samples contain barium in concentrations above analytical detection limits (~0.1 wt.% BaO). In contrast, all four of the colourless marked Lamont bottles that were analyzed are enriched in this component (1.2-2.6% BaO) (Table 1). Since barium, like lead, increases the brilliance of glass, one would expect manufacturers familiar with this ingredient to have included it in glass batches for pressed glass, and not only colourless bottles. Since barium appears to be a signature component in colourless Lamont glass, its absence in all analyzed pattern glass recovered from Glass St. suggests that the pressed glass sherds analyzed were manufactured by another company (i.e., NSGCo). Unless Lamont restricted the use of barium minerals to the manufacture of colourless glass bottles, and omitted this ingredient from the production of other colourless glass objects, it appears that none of the analyzed soda-lime pattern glass or thin-walled etched tumblers can be plausibly attributed to this factory.
31 Although the discovery of a single piece of barium-bearing Trenton pressed glass would reverse the situation, given the available data, we are skeptical of the assignment of any soda-lime tableware to Lamont. However, the reference to “lead glass” in some Lamont advertisements raises the possibility that pressed, alkali-lead glass tableware was produced by this factory. The Ribbed Band creamer (Fig. 2B) analyzed here that contains 9.3 per cent lead oxide (PbO) (Table 1) may be an example. Unfortunately, it does not fluoresce differently than Trenton soda-lime glass, so its recognition requires a spot test for lead, compositional analysis or a density determination. Other pressed glass tableware with an engraved floral motif identical to the decoration on this creamer is known (E. MacArthur, pers. comm. 2005). One example, a “Notched Bullseye” (“Filly”) goblet, also bears the engraved name of Mary Bell, who possibly was related to—or perhaps the wife of—Adam Carr Bell, a shareholder in the NSGCo. It would be worthwhile to analyze more pressed glass with this engraved decoration, to determine if only some (Lamont’s?) consists of alkali-lead glass.
32 There are many examples where tradition and truth are at loggerheads. Contemporary documents concerning mid-19th century glassworks in Vaudreuil County, Quebec, claim that the industry located there because the local sand was “…better than the best that could be had in England…” (cf. Wilson 1996: 28). However, the composition of this sand is completely unsuitable for the production of colourless or pale green glass of the type produced in these glassworks (Owen 2001). It is more likely that Vaudreuil County glass was made from Potsdam sandstone, quarried several tens of kilometers from the glassworks’ sites (Owen and Greenough 2008). Another example concerns the tradition that local red clay (Newlands 1979: 108) probably from the banks of the Conestogo River (Webster 1971: 25) was used to make Eby pottery (ca. 1857-1905). “Clay” from the Conestogo River adjacent to the Eby potworks, however, is actually grey in colour, and a marl (a mixture of clay and lime mud), so its calcium content is inconsistent with the composition of Eby pottery (Owen and Dostal 2006). Thus, any clay used by the Ebys must have been collected elsewhere. Finally, a series of papers by Ross Ramsay and colleagues (2001, 2003) demonstrated the viability of first patent (1744) Bow porcelain (“A”-marked porcelain, London), among (if not the) first such wares made in Britain, and documented the role of an American, Andrew Duché, in this enterprise. Both had previously been discredited for decades (Watney 1973). In a similar vein, Ramsay and Ramsay (2008) have noted that misperceptions in the Western world about the nature of historical Chinese hard-paste porcelain originated with information in letters written in the early 18th century by François Xavier d’Entrecolles. These letters, published in Paris in 1717 and 1722, gave rise to the idea that these wares were derived from firing a mixture of refractory kaolin clay and feldspathic rock (petuntse). This notion has proved to be simplistic (Wood 2000), with the consequence that exactly what is and is not hard-paste or “true” porcelain, as it was subsequently (since the early 18th century) developed in Europe and Britain, is unclear. Analog experiments on first patent Bow (Ramsay et al. 2004) have shed light on this issue (Ramsay and Ramsay 2008).
33 These are just a few examples of how science can help answer questions of history. But what of ours? Did the Lamont Glass Company produce soda-lime pressed glass tableware? It probably did not.
Commentary
34 For far too long, the relationship between the humanities and sciences has been a case of “two solitudes.” In terms of our professional activities, we speak in different languages, use contrasting methodologies, and seemingly pursue unrelated long-term objectives. Yet, the combined expertise in both domains can be mutually beneficial. This is not easily achieved. For example, it can be argued that there is a schism between “connoisseurs” and scientists in the scholarship of early British porcelain. On one hand, many scientists have reason to mistrust seemingly unsubstantiated attributions of artifacts by connoisseurs who base their judgment solely on nebulous visual criteria. On the other hand, most non-specialists lack the technical expertise to fully comprehend papers reporting analytical data for these wares (Pollard 1995). There are, fortunately, exceptions to this generalization. For example, in his Eighteenth Century English Porcelain, George Savage (1952) provides a clear and informative appraisal of the analytical data then available for a variety of British porcelains alongside aesthetic and historical information of these wares.
35 Notwithstanding these rare gems in the literature, there is an ongoing tendency for glaring errors and misperceptions to become entrenched in scholarly papers and thence in the minds of readers. This is an area where the merging of the humanities and sciences can snuff out the promulgation of misinformation. Ross Ramsay and colleagues, for example, have been particularly effective at challenging orthodoxy in the field of high-fired Anglo-British ceramics produced during the mid-18th century. Their strategy has been to objectively assess the literature—in particular primary sources—and to test, wherever possible, statements about particular wares by a combination of analytical data on original pieces, and recreating these objects under controlled conditions. The great advantage of this scientific approach is that hypotheses emerging from an assessment of the historical record can, in many instances, be tested by well-designed experiments. Moreover, the implications can be far-reaching. For example, by demonstrating that American-born Andrew Duché was instrumental in the development of Britain’s early Bow porcelain, Ramsay et al. (2001) have helped changed the mindset of ceramic scholars, many of whom now recognize links between the nascent porcelain manufacturing industry in the United Kingdom and colonial America.
36 In many instances, it is a relatively simple task to sort out some of the contentious issues surrounding nascent industries. For instance, the fact that the first proprietors of Worcester—the longest continuously operating porcelain manufacturer in the U.K. (ca. 1751-present)—experimented with novel pastes and didn’t simply adopt the technology of their short-lived predecessor (Lund’s Bristol, ca. 1749-1751), was established by analyzing sherds from the lowest level of the waster pile at the factory site (Owen 1998).
37 The reasons errors creep into the literature are multifarious. The implications of primary sources can be misconstrued or snippets from these documents can be taken out of context. A good example concerns a ca. 1740s letter containing information pertinent to the Bow porcelain manufactory. Over the years, this undated, primary document, published in full in Daniels (2007), has been variously assigned a date (June 24, 1749), had its author misidentified and the significance of its content unappreciated (Daniels and Ramsay, unpublished data).
38 Archival scholarship aside, misleading information can also be obtained where analogies with modern technologies are drawn for historical artifacts and the methods used to produce them. For example, the temperatures at which historical porcelains were fired have been a source of considerable controversy in recent years. Many modern kaolin-rich porcelain wares are fired at temperatures of between about 1350oC and 1420oC, so some historical true porcelains have been assumed to have been fired at similar conditions (Miller 1998; Owen 2002; Weiss 1971). In contrast, Ramsay and Ramsay (2008) consider that Chinese-type hard-paste wares were likely fired at temperatures somewhat above 1250oC, whereas European soft-paste wares were fired below this temperature. The key to testing this hypothesis involves creating by controlled kiln-firing experiments, analogs of different types (hard-paste and soft-paste) of wares that faithfully replicate historical porcelains in terms of their composition, microstructure, mineralogy and degree of vitrification. To our knowledge, this has yet to be accomplished. Certainly, attempts to construe any information about firing temperatures simply by heating excavated potsherds are flawed because this approach tacitly assumes that ceramic paste mixtures and their fired counterparts respond to elevated kiln temperatures in the same manner (Godden 2004: 152). The firing of a paste mixture representative of first patent Bow porcelain yielded a highly translucent ware that appears to be more highly vitrified than A-marked porcelain itself (Ramsay et al. 2004). Thus it is unclear whether the approximately 1280oC peak temperature used in these experiments is representative of that achieved by Bow. Less highly vitrified A-marked porcelain might either have been soaked at a lower temperature for a longer period of time, or at a similar temperature for a briefer period.
39 In some instances, it is easy to see in an object exactly what we expect. An example of this concerns black glass suspected—based on contemporary advertisements—to have been made at Ontario’s Caledonia Springs glassworks (ca. 1844-1846). Black glass rinds on 19th-century-marked kiln bricks and on rocks found in the vicinity of the glassworks could logically be assumed to be relics of this elusive glass, but analysis of this material shows it to be more aluminous than virtually any historical glass ever produced and, in the case of the glass-on-rock material, it has the same composition of its substrate, which shows clear signs of having been melted (Owen and Culhane 2005). Clearly, preconceived notions can not only distort our reading of the literature, but colour our examination of artifacts as well.
40 The veracity of statements made in historical documents should not be accepted blindly; where they can be tested objectively, they should. Chemical analysis clearly shows that sand from Como and Hudson (Quebec) is insufficiently siliceous to account for the composition of pale-coloured glass produced in the area (Owen 2001). Whence the misleading statements in contemporary newspapers? We can only speculate, but they plausibly were motivated by a desire to build up the glass industry by implying that local ingredients were world-class. In contrast, a careful reading of advertisements in historical periodicals—such as those compiled by Prime (1929)—can lead to fruitful discoveries. For example, advertisements placed in local newspapers by the proprietors of the second known porcelain manufacturer in the United States, hinted at a change in the type of ware they were planning to produce. This change has since been corroborated by the chemical analysis of a dated (1773) porcelain basket (Owen and Hunter 2009). The discovery and recognition of the significance of this artifact also extended the known production history of this short-lived (ca. 1770-1773) factory, which was previously thought to have ceased operations in 1772.
41 Material culture studies are, by their nature, often reliant on opinion, hearsay and variably authoritative documents. We propose that close collaboration with colleagues of diverse expertise will increase the likelihood that factual information be teased out of unreliable sources, and resulting hypotheses tested objectively through analysis and experimentation. This requires objectivity and open-mindedness. Traditional views can be incommensurable with respect to new paradigms (Kuhn 1996), so they tend to die hard, partly because of peer pressure to conform to sanctioned dogma, but also because it is easier to conform to orthodoxy than to challenge it. In the present example, testing the hypothesis that the Lamont Glass Co. produced pressed, soda-lime glass tableware, simply required comparing the chemical compositions of sherds from Glass St. and marked, colourless Lamont bottles. The advantage of this analytical approach is that the conclusion that Lamont probably did not produce this type of pressed glass can itself be tested by seeking barium in more examples of Trenton glass tableware.
Conclusions
42 Pattern glass sherds from Glass St. consist of soda-lime glass with a wide range of soda-lime ratios; a small minority of samples has magnesian compositions and none contains barium above analytical detection limits. Neither do thin-walled, etched tumblers of the type traditionally assigned to the Lamont glassworks. Barium does occur, however, in substantial concentrations (1.2-2.6% BaO) in marked, colourless Lamont bottles. Its absence in examples of eighteen different pressed glass patterns recovered from Glass St., including one (Crown) often attributed to Lamont, suggests that none of this glass was produced by Lamont. Unless Lamont excluded barium minerals from their colourless, soda-lime glass batches, it therefore seems unlikely that they produced tableware in this medium. It is possible, however, that Lamont produced alkali-lead glass tableware (the analyzed Ribbed Band creamer in Table 1) since their advertisements do make reference to “lead glass” as a specialty. The term “lead glass” may also refer to cut glassware.
43 Future work should focus on archival records and contemporary advertisements, and the analysis of more marked Nova Scotia glass artifacts, or glass with the distinct engraved floral decoration seen on the alkali-lead glass, Ribbed Band creamer described here. In a broader sense, we advocate closer collaboration between researchers in the humanities and sciences, so that objective and innovative solutions to problems related to material culture can be achieved.
Note
This project could not have been completed without the generosity of numerous glass enthusiasts who provided both samples and information. Ed MacArthur of New Glasgow provided much useful and informative discussion about Trenton glass. He also generously allowed the first author to microsample specimens of both cullet and some intact objects (a goblet and a glass cane) in his Antiques shop, and to browse through his copy of the elusive Barclay (1987) reference. Sid Lethbridge alerted us to the 1881 Monetary Times reference to Berkshire sand. Bob Cunningham provided a fragment from his marked Humphrey’s bottle. Billy Dwyer made available particles of a glass cane originally owned by his great aunt, Donald Lamont’s sister. Sheila M. Moores graciously permitted the microsampling of intact specimens of Nova Scotia glass in her Canadiana collection. All of these individuals are thanked for their interest in this project. Analytical work was supported by an NSERC Discovery grant to J. Victor Owen. The manuscript benefited from comments by Ross Ramsay and an anonymous referee from Material Culture Review/Revue de la culture matérielle.References
Barclay, J. C. 1987. The Canadian Friut (sic) Jar Report: An Illustrated Book of Canadian Fruit Jars and Produce Jars. Book 2; issue 2. Ridgetown, ON: Dominion Press Ltd.
Daniels, Pat. 2007. The Origin & Development of Bow Porcelain 1730-1747, Including the Participation of the Royal Society, Andrew Duchè and the American Contribution. Oxfordshire: Resurgat Publishing.
Godden, Geoffrey A. 2004. Godden’s New Guide to English Porcelain. London: Miller’s.
King, Thomas B. 1987. Glass in Canada. Erin, ON: The Boston Mills Press.
Kuhn, T. 1996. The Structure of Scientific Revolutions. Chicago: University of Chicago Press.
MacLaren, George. 1971. Nova Scotia Glass. Halifax, NS: Nova Scotia Museum.
Miller, J. 1998. Miller’s Antiques Encyclopedia. London: Miller’s.
Newlands, David L. 1979. Early Ontario Potters. Their Craft and Trade. Toronto: McGraw-Hill Ryerson.
Owen, J. Victor. 1998. On the Earliest Products (c. 1751-52) of the Worcester Porcelain Manufactory: Evidence From Sherds From the Warmstry House Site, England. Historical Archaeology 32 (4): 60-72.
———. 2001. The Como-Hudson Factories (c. 1845-77): Results of Geochemical Analyses for Quebec’s First Known Glassworks. Canadian Journal of Archaeology 25 (1-2): 74-97.
———. 2002. Antique Porcelain 101: A Primer on the Chemical Analysis and Interpretation of Eighteenth-Century British Wares. In Ceramics in America, ed. Robert Hunter, 39-61. Milwaukee: Chipstone.
———. 2003. Geochemical Characteristics of Alleged Mallorytown Glass (c. 1839-40) in the Royal Ontario Museum and Its Distinction From Contemporary Upstate New York Glass-ware. Canadian Journal of Archaeology 27 (2): 287-308.
Owen, J. Victor and Phillip Culhane. 2005. Pyrometa-morphism of 19th Century Kiln Artifacts From Caledonia Springs, Ontario, Canada. Geoarchaeology 20 (8): 777-96.
Owen, J. Victor and Jaroslav Dostal. 2006. Phase Equilibrium Constraints on Kiln Temperatures at the Eby Pottery (ca. 1857-1905). Conestogo, Ontario. Canadian Mineralogist 44 (5): 1257-1266.
Owen, J. Victor and John D. Greenough. 2008. Influence of Potsdam Sandstone on the Trace Element Signatures of Some 19th century American and Canadian Glass: Redwood, Redford, Mallorytown, and Como-Hudson. Geoarchaeology 23 (5): 587-607.
Owen, J. V. and R. Hunter. 2009. Too Little, Too Late: The Geochemistry of a 1773 Philadelphia Porcelain Openwork Basket. Journal of Archaeological Sciences 36 (2): 333-42.
Owen, J. Victor, Kathleen L. Irwin, Charles L. Flint and John D. Greenough. 2005. Trace Element Constraints on the Source of Silica Sand Used By the Boston and Sandwich Glass Co. (ca. 1826-1888), Massachusetts. Industrial Archaeology 31 (2): 39-56.
Pierce, Lorne. 1958. Early Glass Houses of Nova Scotia. Toronto: Ryerson Press.
Pollard, A. M. 1995. Why Teach Heisenberg to Archaeologists? Antiquity 69 (263): 242-47.
Prime, A. C. 1929. The Arts and Crafts in Philadelphia, Maryland, and South Carolina, 1721-1785: Gleanings from Newspapers. Massachusetts: Wayside Press.
Ramsay, W. Ross H. and Elizabeth G. Ramsay. 2008. A Case For the Production of the Earliest Commercial Hard-Paste Porcelains in the English-Speaking World by Edward Heylyn and Thomas Frye in About 1743. Proceedings of the Royal Society of Victoria 120 (1): 234-54.
Ramsay, W. Ross H., Anton Gabszewics and Elizabeth G. Ramsay. 2001. Unaker or Cherokee Clay and Its Relationship to the “Bow” Porcelain Manufactory. English Ceramic Circle: Transactions 17:474-99.
———. 2003. The Chemistry of “A”-Marked Porcelain and its Relation to the Edward Heylyn and Thomas Frye Patent of 1744. English Ceramic Circle: Transactions 18:264-83.
Ramsay, W. Ross H., G. R. Hill and Elizabeth G. Ramsay. 2004. Re-creation of the 1744 Heylyn and Frye Ceramic Patent Wares Using Cherokee Clay: Implications For Raw Materials, Kiln Conditions, and the Earliest English Porcelain Production. Geoarchaeology 19 (7): 635–55.
Savage, George. 1952. Eighteenth Century English Porcelain. London: Rockliff.
Spence, Kelvin and Hilda Spence. 1972. A Guide to Early Canadian Glass. Toronto: Longman.
Stevens, Gerald. 1967. Canadian Glass c. 1825-1925. Toronto: Ryerson Press.
———. 1979. Early Canadian Glass. Toronto: Coles.
———. 1982. Glass in Canada. Toronto: Metheun.
Unitt, Doris and Peter. 1969. Treasury of Canadian Glass. Peterborough, ON: Clock House.
Watney, Bernard. 1973. English Blue & White Porcelain of the Eighteenth Century, 2nd ed. London: Faber and Faber.
Webster, Donald B. 1971. The William Eby Pottery, Conestogo, Ontario 1855-1907. Toronto: Royal Ontario Museum.
Weiss, G. 1971. The Book of Porcelain. Trans. J. Seligman. New York: Praeger.
Wereley, Chris. 2003a. Donald Lamont, the Lamont Glass Co., and the L.G. Co. Fruit Jars. Canadian Bottle and Stone-ware Collector 7 (4): 4-13.
———. 2003b. Nova Scotia Jars: The “Scotia” and the “Beaver.” Canadian Bottle and Stoneware Collector 8(3): 10-15.
Wilson, Olive I. 1996. Glass in Vaudreuil, Lower Canada, 1845-1877. Quebec: Musée régional du Vaudreuil-Soulanges.
Wood, Nigel. 2000. Plate Tectonics and Chinese Ceramics: New Insights Into the Origin and Distribution of China’s Ceramic Raw Materials. Taoci 1:15-24.