Research Reports / Rapports de recherche
Metallurgic Analysis of Slag Samples from a Seventeenth-Century Blacksmith Shop in Ferryland, Newfoundland
1 Excavation of a blacksmith shop at Ferryland, Newfoundland, revealed an almost complete stone forge, a blacksmith's vise, a hammer, shears, files, several examples of iron tools in for repair and a variety of other material culture dating from the first half of the seventeenth century. Included in this deposit was a large accumulation of slag (a by-product of blacksmithing) and a heavily concreted mass of forge clinker, dirt and iron fragments surrounding the area that once held the anvil. A metallurgical analysis of the slag can help us to ascertain aspects of productivity and the quality of work that the blacksmiths produced. The iron and soil concretion proved to be informative in terms of the small finds. Included in this mass was a wooden tuning peg preserved in tire corrosion layer. This artifact, in addition to the many shards of bottle glass, indicates a secondary use of the forge as a place of relaxation and merriment.
2 From a conservation perspective, one of the challenges for this feature was the stabilization of large sections of concreted soil and dirt and the numerous diagnostic smithing tools. These objects required hours of chemical and mechanical cleaning. In addition, scientific analysis, including x-radiography and wavelength dispersive x-ray fluorescence (XRF), revealed physical attributes and chemical composition of the objects studied.
3 Slag originating from both the smithy and another early seventeenth-century deposit, sixty metres from the first, was analysed for chemical composition by XRF. The results were used to determine if the slag from both areas are of the same chemical composition. If this is not the case, then it suggests that there was another blacksmith shop operating at Ferryland during the first half of the seventeenth century. The chemical analysis could be used as a baseline or reference point for researchers examining slag from other blacksmith sites.
The Ferryland Blacksmith Shop
4 The community of Ferryland is located on Newfoundland's Avalon Peninsula approximately eighty kilometres south of St John's. In 1621 George Calvert, the first Lord Baltimore, sent a group of twelve men, including Captain Edward Wynne, to begin construction on the colony of "Avalon" at Ferryland. In Wynne's correspondence to Calvert, July 28, 1622, he makes particular reference to the colony's forge that "hath been finished this five weeks."1 Another letter dated August 17, 1622, states that there were two blacksmiths, Thomas Wilson and John Prater, working at Ferryland. These are the only two documentary references to a blacksmith shop at Ferryland and therefore we must rely on the archaeological record to develop a more complete picture of the early operations of this forge.
5 Initial test excavations, in 1984, uncovered portions of the forge, but it was not until 1994, when financial and conservation support was made available, that complete excavation was possible. These excavations revealed that the early blacksmith shop was constructed directly into the side of a hill that flanks much of the original colony. By digging into the hillside, the colonists were able to "reclaim previously void or waste ground," on which to build the forge and use the same earth to generate new land along the shoreline. The wooden walls of the blacksmith shop were positioned directly against the sub-soil, with the exception of the front wall, and the total dimensions of the building measured 3.5 metres by 5.25 metres. The central feature of this structure was a well-preserved rectangular stone forge (Fig. 1). Artifactual remains within the shop revealed that the blacksmiths performed a variety of tasks including gunsmithing, locksmithing, coppersmithing and farriering as well as blacksmithing.2
6 The time period in which the blacksmith shop was in use was determined through an examination of the ceramic, glass and clay tobacco pipe fragments found within. Matthew Carter's analysis of the artifacts has established that the forge was indeed the one mentioned by Wynne in 1622 and that it remained functional until sometime into the 1640s to 1650s.3
7 Over 950 pieces of slag were excavated from the blacksmith shop. An additional 700 pieces of slag were discovered during the excavations of a waterfront storehouse, Area C, constructed between 1623 and 1629.4 Examination of the stratigraphy in this area revealed that the layer containing the slag pre-dated the waterfront construction and that it was part of a single fill episode. This suggests that the deposit of slag and refuse originated from the blacksmith shop. Artifactual remains alone could not substantiate this finding as they would all represent a similar time period. Chemical analysis of the slag composition, however, could provide the necessary supporting information. If slag composition from the two areas matched we could be certain that they did originate from the same forge.
XRF Analysis
8 An iron ore deposit can contain many impurities including silicates, carbonates and oxides.5 These were generally considered undesirable by-products and were removed, usually at the bloomery. Any remaining impurities could be removed by the blacksmith as a slag by-product of working the iron. Slag can generally be described as a mixture of non-combustible or glassy material in the coal/charcoal matrix.6 Some of the slag was incorporated into the final product as inclusions. During the working of the iron, slag inclusions would be stretched and thinned and transformed from a liquid to crystalline state.7 Carter has determined that the source of fuel for the Ferryland forge was probably charcoal and coal, introducing carbon, phosphorous and sulphur to the iron.8 Slag found at the site could provide information about the impurities in the Ferryland manufactured iron artifacts.9 If the two slag heaps found at Ferryland were both from the same forge, the type and concentration of impurities should be similar.
9 For the preliminary investigation forty-six pieces of slag were selected with thirty pieces being from the blacksmith shop (Area B) and sixteen pieces from the early seventeenth-century fill deposit sixty metres away (Area C). Overall the physical shape of the slag from both areas was similar with dimensions averaging 9.9 centimetres by 7.7 centimetres by 5.1 centimetres. Most pieces of slag were caked-shaped having both a convex base and a concave upper surface. Unglik and Light believe that slag may have acquired this particular shape from the bowl-shaped forge bottom.10 The upper surface of the slag was smooth and glassy whereas the bottom surface was rough. The colour of the slag varied from light to dark grey and brown. A small percentage of the slag samples (five percent) had fragments of coal embedded in their surface. None of the slag samples exhibited any considerable ferromagnetism. Both clusters of slag physically resemble hearth slag. This type of slag is described as an accumulation of slag droplets and scale, and other material such as metallic iron, from the base of the smithing hearth.
10 Slag samples were pulverized to 200 mesh and prepared as pressed powder pellets using BRP-5933 Bakelite phenolic resin as a binding agent. The pellets were analyzed for thirty elements (Al, As, Ba, Ca, Ce, Cl, Cr, Cu, Fe, Ga, K, Mg, Mn, Na, Nb, Ni, P, Pb, Rb, S, Se, Si, Sr, Th, Ti, U, V, Y, Zn and Zr) using a Fisons/ARL model 8420 sequential wavelength dispersive x-ray spectrometer. Detection limits range from 100 parts per million for the light major elements such as Na and Mg down to 0.6 to 0.7 parts per million for the less abundant elements of Rb, Y and Nb.11 To check accuracy of the calibration technique we analysed an iron ore standard (Lincolnshire Iron Ore deposit — Bureau of Analysed Samples, Ltd No. 301) during all analytical sessions. Results were then compared with the known values for the standard.12
11 It was determined early in this study that there was no reference material, i.e. standard, available for quality control purposes that was an exact matrix match for the slag material. To ensure the best quality data possible the authors undertook to develop an in-house reference material that could be used in this study and any future work as a measure of precision and accuracy. A sufficient volume of composite Ferryland slag was pulverized and homogenized. Pressed pellets prepared from this material were run with all analytical sessions and the results used as a measure of reproducibility between analytical sessions. It is hoped that future slag projects using other analytical techniques will utilize this material to determine additional elements and verify the numbers determined by this study.
X-ray Fluorescence Spectrometry (XRF) Results
12 The XRF analysis determined there was no significant discrepancies between the slag that originated from the smithy and that found at the southwest corner of Area C. Tables 1 and 2 summarize the results and present the mean, standard deviation and spread for the data. The chemical analyses for the slag from Areas B and C had a similar standard deviation indicating that slag composition was essentially the same for both areas. Consequently, when using the XRF data in conjunction with that obtained through the analysis of the artifacts found in association with the two clusters of slag, one can suggest that the Area C slag likely originated from the Area B blacksmith shop. No evidence was uncovered that would substantiate the existence of a second blacksmith shop at Ferryland.
13 The Ferryland slag sample did not contain many pieces that could be classified as being contaminated. Contaminated slag, according to Light and Unglik, is inhomogeneous in nature, porous, light in colour and weight.13 True slag is consistent throughout in terms of concentration and physical appearance, dark in colour and heavy. It was determined that only three of the forty-six Ferryland slag samples fit into this classification, two originating from Area B and one from Area C. These three samples had silicate and iron oxide concentrations of 30.64% and 17.63% respectively for Area B and 20.57% and 30.43% for Area C, respectively. Tables 1 and 2 show the average silicate and iron concentrations to be 19.38% and 33.65% respectively. Contaminated slag is possibly formed when the forge lining becomes incorporated into the molten slag. According to Light and Unglik, finding a high proportion of contaminated slag suggests a low state of technology either because of inferior building materials for the forge or lack of proper maintenance of the forge by the smith.14 Given that only three of the Ferryland slag samples fit into the contaminated slag classification it seems that the smiths who operated at Ferryland maintained their forge. Also the slag itself contained relatively few fragments of iron objects, which is another indicator of competent work.
14 The analysis also provides evidence for a concentration of copper in the samples. Anomalies in the data (removed for the purposes of statistical analysis) indicate that a few slag samples had as much as 27,889 parts per million of copper suggesting that the smiths were involved in the working of copper or copper alloys (i.e., brazing).
Excavation and Conservation of the Forge Floor Concretion
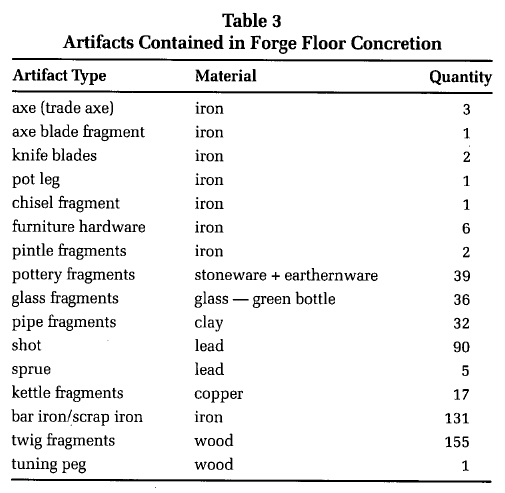
15 During the field season of 1994 a section of the forge floor was removed for analysis and conservation. The artifact measured approximately 69 centimetres long, 51 centimetres wide and 10 centimetres thick. The concrete nature of the object prevented further excavation in the field laboratory. It was therefore decided to soften the ferrous matrix by immersion in a sodium hydroxide solution. As this section was located from around the anvil base, analysis of the artifacts within the clinker/iron corrosion concretion could provide a glimpse of the blacksmith's activities. A separate tank for treatment and many hours of conservation were dedicated to this object because of its potential value for interpreting the past.
16 X-radiograph analysis conducted on the concretion provided evidence for artifactual remains being held in the concretion. It was decided to chemically stabilize the concretion to prevent further corrosion and mechanically remove, where possible, identifiable objects. Immersion of the concretion in an aqueous one percent (w/v) sodium hydroxide solution was conducted to facilitate both chloride removal (for iron artifact stabilization) and artifact removal from the concretion. Treatment continued for five years with regular changes of solution (six changes per year). Following chemical cleaning, the object was rinsed with tap water to remove residual sodium hydroxide and air dried. Mechanical cleaning to remove artifacts from the concretion was conducted using pin vices and vibro tools. Table 3 presents the artifacts that were excavated from the forge floor concretion.
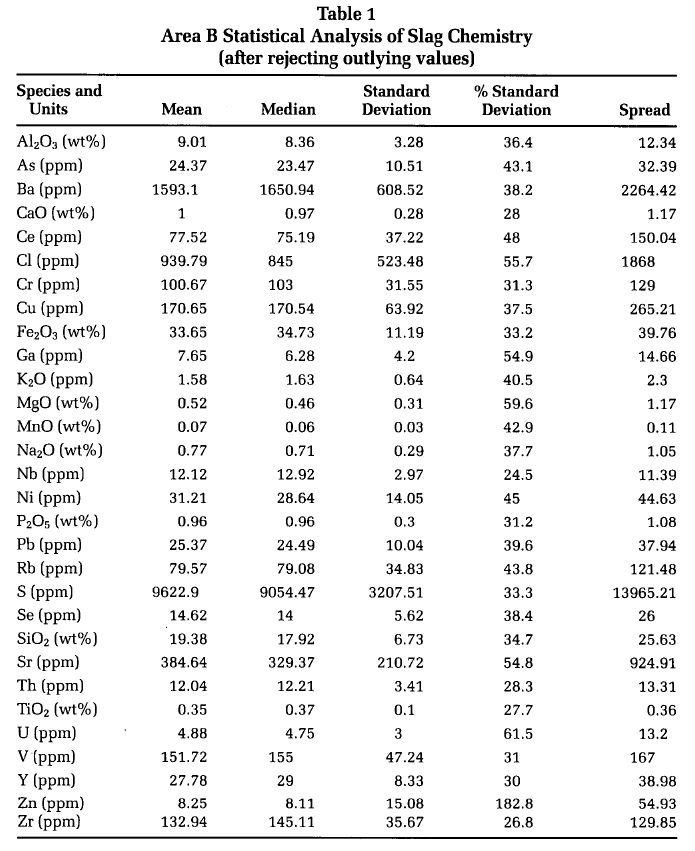 Display large image of Table 1
Display large image of Table 1 Display large image of Table 2
Display large image of Table 217 The domestic refuse (ceramics, pipes, bottle glass and tuning peg) found on the forge floor provides evidence for the social activities occurring within the blacksmith shop. Given the nature of Newfoundland weather it is not surprising that on those cold, windy days the forge provided a refuge for all who dared to enter. The axes, knife blades, chisels, furniture hardware and bar iron provide evidence to the extensive use of the forge during the first part of the seventeenth century. The copper kettle fragment provides further evidence for copper smithing.
Conclusion
18 X-ray fluorescence analysis conducted on slag remains found at Ferryland indicate that a single forge provided the needed services for the colony during its building phases. Examination of the slag has also provided some interesting ideas about the forging practices of the resident blacksmiths. Since few of the slag pieces were "contaminated" it can be suggested that the blacksmiths maintained a clean fire. Also, the fact that few iron object fragments were found in the slag samples demonstrates that the smiths were very competent at their work. One could conclude that this early manufacturing centre was a success in terms of quality of production during its history of operation. In addition, the XRF analysis provided evidence that suggests that the smiths frequently worked with copper.
19 Domestic artifactual remains found when excavating a concreted mass from the forge floor provides insight into the working conditions at this time. It would appear that the Ferryland forge was, at times, a site for warmth and merriment.
20 This type of analysis is extremely valuable since it can reveal a substantial amount of information about the working habits of blacksmiths that could not be found through the examination of artifacts by themselves. This information also adds to our overall understanding of the material culture for an English colonial site in the seventeenth century.
Future Work
21 The conservation of metal artifacts from both Areas B and C are now complete. Memorial University's Conservation Department in partnership with the Canadian Conservation Institute will be conducting a survey of treatments to determine the best approaches for stabilizing material culture made from iron. As of 2003 the project has generated a wealth of iron tools and hardware dating from the seventeenth century. We welcome researchers interested in assisting in the analysis of these materials.