Series
Great Canadian Lagerstätten 4.
The Devonian Miguasha Biota (Québec): UNESCO World Heritage Site and a Time Capsule in the Early History of Vertebrates
http://dx.doi.org/10.12789/geocanj.2013.40.008
SUMMARY
Over the past 170 years, the Late Devonian Miguasha biota from eastern Canada has yielded a diverse aquatic assemblage including 20 species of lower vertebrates (anaspids, osteostra-cans, placoderms, acanthodians, actinopterygians and sarcopterygians), a more limited invertebrate assemblage, and a continental component including plants, scorpions and millipedes. Originally interpreted as a freshwater lacustrine environment, recent paleontological, taphonomic, sedimentological and geochemical evidence corroborates a brackish estuarine setting. Over 18,000 fish specimens have been recovered showing various modes of fossilization, including uncompressed material and soft-tissue preservation. Most vertebrates are known from numerous, complete, articulated specimens. Exceptionally well-preserved larval and juvenile specimens have been identified for 14 out of the 20 species of fishes, allowing growth studies. Numerous horizons within the Escuminac Formation are now interpreted as either Konservat– or Konzentrat–Lagerstätten.
SOMMAIRE
Au cours des 170 dernières années, le biote du Dévonien supérieur de Miguasha de l’Est du Canada a fourni un assemblage aquatique diversifié, comprenant 20 espèces de vertébrés inférieurs (anaspides, ostéostracés, placodermes, acanthodiens, actinoptérygiens et sarcoptérygiens) et un assemblage peu diversifié d’invertébrés ainsi qu’une composante continentale, représentée par des plantes, des scorpions et des mille-pattes. À l’origine interprété comme un milieu lacustre d’eau douce, les dernières preuves paléontologiques, taphonomiques, sédimentologiques et géochimiques confirment un environ-nement saumâtre rappelant celui d’un estuaire. Plus de 18,000 fossiles de poissons ont été découverts montrant différents états de conservation, notamment en trois dimensions et la préservation de tissus mous. La plupart des vertébrés sont connus par de nombreux spécimens complets et articulés. Des spécimens de larves et de juvéniles, exceptionnellement bien conservés, ont été identifiées pour 14 des 20 espèces de poissons permettant des études détaillées de leur croissance. De nombreux horizons au sein de la Formation d’Escuminac sont inter-prétés soit comme des Konservat– ou Konzentrat–Lagerstätten.
HISTORICAL OVERVIEW
1 The Miguasha fossil site in eastern Québec was among the first major paleontological localities to have been discovered and excavated in North America. The discovery of the first fossils at Miguasha was made in 1842 by Abraham Gesner, the government geologist in New Brunswick, also known for his work on the distillation of kerosene. While surveying the northern part of New Brunswick for coal, Gesner came to Miguasha and reported “I found the remains of fish, and a small species of tortoise with foot-marks” (Gesner 1843, p. 64). Evidently, this fossil was not a tortoise but rather a placoderm fish, most likely Bothriolepis canadensis, one of the most common fish from the Escuminac Formation. Although fossil plants were found, there was no potential in terms of coal mining, and the site was forgotten for more than 30 years.
2 Between 1879 and 1881, the Geological Survey of Canada organized several expeditions to Miguasha lead by R. W. Ells (Fig. 1a), A. H. Foord and T. C. Weston. A few dozens of collected fossils were given to pioneer paleontologists: Joseph F. Whiteaves, a British paleontologist working at the Geological Survey of Canada, who studied the fossil fishes, and Sir J. William Dawson, a Canadian paleobotanist working at McGill University, who looked at the fossil plants. These workers were the authors of the first scientific descriptions of the Miguasha fossils (Whiteaves 1880; Dawson 1882). From the late 1880s until the 1940s, British and American paleontologists came to Miguasha (frequently referred to mistakenly as Scaumenac Bay) in order to collect new material for major museums, such as the British Museum of Natural History (London, England), the Royal Scottish Museum of Edinburgh (Scotland) and the American Museum of Natural History (New York, USA). From 1887 to 1892, M. Jex collected an impressive array of fossil fishes in Miguasha, which he sold to different museums in the United Kingdom. As a result, part and counterpart of the same fossils were sold separately to paleontological collections in Edinburgh and London. From this fossil hunting, four fish species were named by two famous British paleontologists, R. H. Traquair and A. S. Woodward.
 Display large image of Figure 1
Display large image of Figure 13 In 1892, the American vertebrate paleontologist E. D. Cope was the first to recognize that the osteolepiform Eusthenopteron foordi from Miguasha had a fin anatomy similar to that of the limbs of stegocephalians (Cope 1892), a paraphyletic group acknowledged today to include stem tetrapods. Fossils from Miguasha thereby made their entrance to studies documenting the origin and evolution of major groups of vertebrates, a perspective fairly new at the time, considering the Darwinian revolution. Following Cope’s (1892) publication, Eusthenopteron foordi was considered a key species in the transition from fishes to tetrapods, thus promoting the focus of numerous studies on the anatomy of its paired fins, vertebral column and nostrils. Between 1905 and 1993, local collectors (Fig. 1b–c) familiar with fossil fish hunting in Miguasha were pivotal in creating reference collections now available around the world (Lemieux 1996). In the early 1920s, Swedish paleontologists started to study in great detail the anatomy of fishes from Miguasha, contributing, in part, to recognition of the famous ‘Swedish School’ of paleozoology at the Naturhistoriska Riksmusett in Stockholm (Schultze 2009). Between 1937 and 1998, Erik Jarvik wrote some thirty scientific articles on Eusthenopteron (Cloutier 1996c), while Erik Stensiö published on the detailed anatomy of the placoderm Bothriolepis (Stensiö 1948).
4 The history of the Miguasha biota can also be tracked through the sequence of scientific publications and the date of original descriptions for the various vertebrate species (Fig. 2). From 1880 to 1900, half of the known vertebrate diversity had been described on the basis of original collecting in Miguasha. Between 1900 and 1924, most likely as a result of global social instability and World War I, paleonto-logical research, including that on the Miguasha biota, was not a priority in the scientific community. Renewed interest in the fossil material collected in Miguasha by British, Swedish and American paleontologists stimulated a burst of taxonomic descriptions in the mid to late ‘30s, which eventually faded out with World War II.
 Display large image of Figure 2
Display large image of Figure 25 Since 1960, a great deal of attention has been given to the evolutionary interpretation of the Miguasha fossil fishes. Reinterpretation of the fossil material in various collections resulted in the fluctuation of species-level diversity figures until a thorough revision of the Miguasha biota was put together in 1996 (Schultze and Cloutier 1996). Emphasis then shifted from a descriptive perspective to an interpretive one, where the phylogeny, the paleoenvironment, and the taphonomy became important research focuses, and it was at this time that Miguasha was perceived as a Lagerstätte. For the past 168 years, fossils from the Miguasha biota have contributed greatly to our understanding of the early evolution of vertebrates. Over the years, at least 367 scientific papers (Fig. 2) have been published by 230 authors from 25 countries. As a modern indicator of interest, more than 200,000 pages on the internet mention fossils from Miguasha.
6 Aware of the international interest in Miguasha fossils, the Government of Québec created a conservation park in 1978 to protect the Escuminac Formation. Starting in the 1990s, this initiative stimulated renewal of interest in fish paleontology among Canadian researchers who received their Ph.D. training either in Canada (F. Charest), the USA (R. Cloutier) or in France (M. Belles-Isles, P.-Y. Gagnier, D. Vézina), and who studied different perspectives of the Devonian Miguasha biota. In 1999, the Miguasha National Park was confirmed as a UNESCO World Heritage Site in recognition of its global status as the best representation of the Devonian ‘Age of Fishes’ (Cloutier and Lelièvre 1998).
7 Over the past 20 years, the focus on the biota has shifted from detailed morphological studies, to the phylogenetic positioning of the Miguasha taxa, to the paleoenviron-mental reinterpretation of the Escuminac Formation, to studies of exceptional preservation, including documentation of numerous fossilized ontogenies. In combination, these studies make the Miguasha biota one of the most intensively investigated paleontological sites anywhere.
GEOLOGICAL SETTING
8 Miguasha National Park is located in eastern Québec on the south coast of the Gaspé Peninsula along the estuary of the Restigouche River (Fig. 3a). The Miguasha biota is contained in the Late Devonian Escuminac Formation (119 m thick), which outcrops primarily as a cliff (from three to 30 m high) along the Restigouche River. Additional satellite outcrops, located between 3 and 40 km away from the main cliffs, have been discovered over the past ten years (Fig. 3c). In 2007, the main Miguasha exposures (Fig. 3b) were named the ‘René Bureau Cliffs’ to honour a self-taught geologist and paleontologist (Fig. 1c) whose work in the 1930s and 1950–1970 was pivotal in establishing this site as a Québec conservation park.
9 The Escuminac Formation conformably overlies the Fleurant Formation (conglomerate containing sandstone lenses); together, these units constitute the Miguasha Group. Rust et al. (1989) interpreted the Fleurant Formation as proximal alluvium deposits. The Lower Carboniferous Bonaventure Formation (alternating conglomerate and coarse sandstone) rests disconformably on the Escuminac Formation and are interpreted to represent proximal alluvial fan deposition (Rust et al. 1989).
10 The Escuminac Formation is considered to be middle Frasnian in age, or 385 to 374 Ma according to the timescale of Walker and Geissman (2009), based on miospore content and the fish assemblage (Cloutier et al. 1996; Elliott et al. 2000). Although a precise and absolute dating of the duration of the Escuminac Formation is not possible, the timespan is estimated to lie between 59.5 ka and 2500 ka (Cloutier et al. 2011).
11 Numerous sedimentological descriptions of the Escuminac Formation show the importance of the alternation between siltstone – sandstone and shale (Alcock 1935; Russell 1939; Dineley and Williams 1968a, b; Carroll et al. 1972; Hesse and Sawh 1982, 1992; Vézina and Cloutier 1991; Prichonnet et al. 1996; Cloutier et al. 2011), and suggest that the sequence comprises lacustrine or estuarine turbidite deposits (Hesse and Sawh 1992; Prichonnet et al. 1996). The clear alternation of lithology allows recognition of 394 individually numbered horizons from the base to the top of the Escuminac Formation at René Bureau’s Cliffs (Fig. 3d). As a result of such precise stratigraphic positioning, systematic collecting has enabled documentation of the stratigraphic distribution of various Escuminac species (Cloutier et al. 1996; Cloutier et al. 2011) and of taphonomic modes (Parent and Cloutier 1996; Cloutier et al. 2011).
 Display large image of Figure 3
Display large image of Figure 3PALEOBIOLOGY
Diversity of the Miguasha Biota
12 The diversity of the Miguasha biota includes an allochthonous continental component, composed of plants and invertebrates, and an autochthonous aquatic component, composed of invertebrates and lower vertebrates (Fig. 4). Foliages of various continental plants, e.g. Archaeopteris (Fig. 4a), Spermasporites, Protobarynophyton, Barynophyton and Flabellofolium (Gensel and Barnett-Lawrence 1996) are found in several strata; some are very abundant (Cloutier et al. 2011). In addition to the plant macrofossils, a wide range of spores (more than 70 species) and marine acritarchs (15 genera) have been identified (Cloutier et al. 1996). The diverse miospore assemblage could suggest long distance fluvial transport, which is characteristic of equatorial to tropical phytogeographic zones (Cloutier et al. 1996).
13 Invertebrates are represented by 12 species and account for a minor part of the diversity of the Miguasha biota. The aquatic component of the invertebrate fauna includes the spinicaudatan (or conchostracan) Asmusia membranacea (Martens 1996; Fig. 4h), a parastylonurid eurypterid (Jeram 1996), and a scolecodont (Cloutier et al. 1996); the continental component includes the millipede Zanclodesmus willetti (Wilson et al. 2005; Fig. 4b), the scorpion Petaloscorpio bureaui (Fig. 4c), and a gigantoscorpionid (Jeram 1996). Fragments of arthropod cuticles, some of them likely referable to arachnids or trigonotarbids (Cloutier et al. 2011), have been found in palynological preparations (Cloutier et al. 1996). Two aquatic ichnotaxa (Fig. 4f–g) have been identified (Maples 1996; Schultze 1999), as well as an additional two types of undescribed traces. Noteworthy is the total absence of typical marine invertebrates, a fact which led earlier researchers (Dineley and Williams 1968a, b) to interpret the Miguasha paleoenvironment as freshwater lakes.
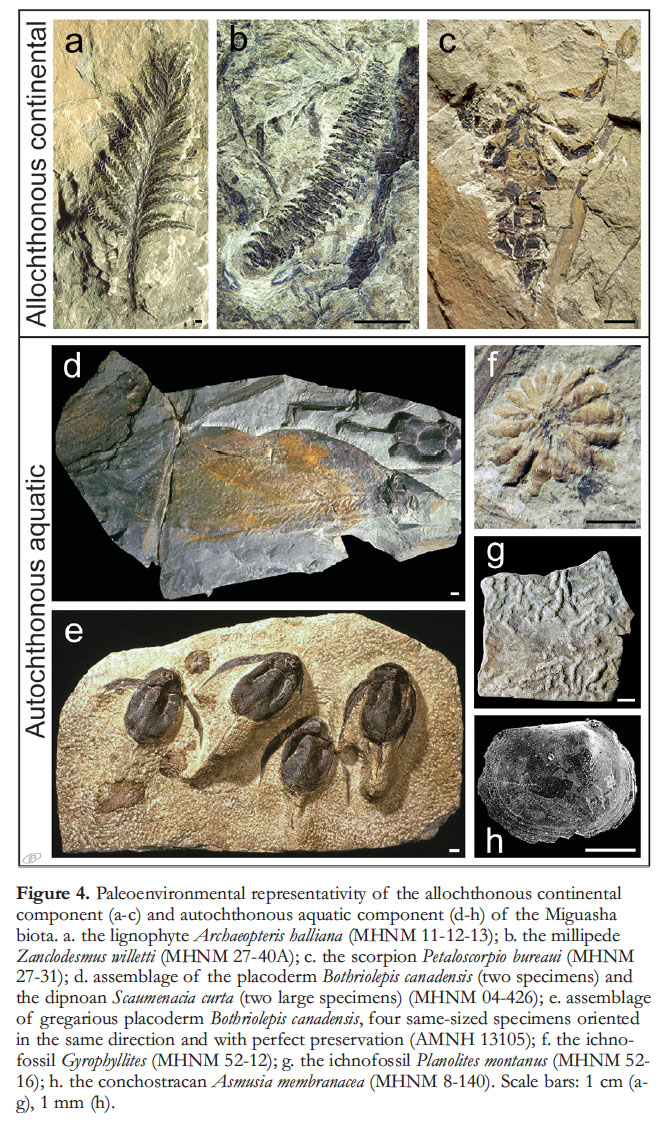 Display large image of Figure 4
Display large image of Figure 414 The Escuminac Formation owes its scientific reputation largely to its vertebrate fauna. This assemblage currently includes 20 species representative of ten major groups of lower vertebrates (Table 1). The recent discovery of new specimens of the taxon referred to as holoptychiid sp. indet. (Cloutier and Schultze 1996) or Porolepiformes indet. (Cloutier et al. 2011) confirms that it corresponds to a new species of holoptychiid, which is currently under study. The diversity of Miguasha fishes spans a phylogenetic breadth from some of the most basal vertebrates (the anaspids) to some of the most derived forms (the elpistostegalians). When compared to a survey of 180 Devonian vertebrate assemblages around the world (Cloutier et al. 2011), the richness of the Miguasha biota is significantly above the average of approximately four species (Fig. 5).
 Display large image of Figure 5
Display large image of Figure 5Fossil Abundance of the Miguasha Biota
15 The fossil assemblage is not only fairly diverse, but it is abundant. More than 18,000 specimens of fishes have been found in the Escuminac Formation since its initial discovery. The relative abundance varies greatly among taxa (Fig. 6): some species are known only from a few specimens, whereas others, such as the placoderm Bothriolepis canadensis, the acanthodian Triazeugacan-thus affinis, the dipnoan Scaumenacia curta and the osteolepiform Eusthenopteron foordi, are each known from thousands of specimens. The placoderms, most belonging to Bothriolepis, are the most abundant fish group, representing ca. 38% of the specimens, most of them belonging to Bothriolepis. Five species are extremely rare: Callistiopterus clappi (one specimen), Elpistostege watsoni (four specimens including one isolated scale), the new holoptychiid species (four specimens), Diplacanthus ellsi (six specimens), and Levesquaspis patteni (nine specimens). The taxonomic validity of C. clappi, known from a single fairly complete ‘juvenile’ specimen (Thomson and Hahn 1968), has been questioned. Overall, the conchostracan Asmusia is the most abundant fossil present, and bedding surfaces at numerous horizons are covered by masses of their tiny (2-4 mm wide) valves.
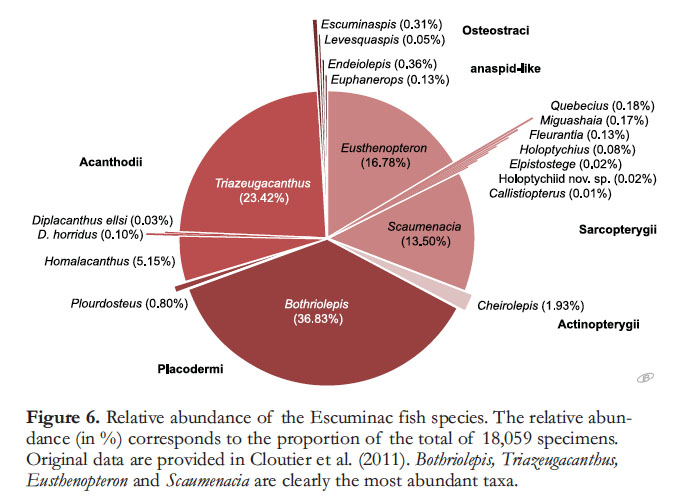 Display large image of Figure 6
Display large image of Figure 6Evolutionary Significance of the Miguasha Biota
16 Numerous Escuminac species are recognized for their evolutionary and phylogenetic significance (Schultze and Cloutier 1996; Cloutier 2009). Among the plants, the lignophyte Archaeopteris (Fig. 4a; classically identified as a progymnosperm) is considered the sister group of the gymnosperms and might have constituted some of the oldest forests in the fossil record (Meyer-Berthaud et al. 1999). Although extremely rare, Spermasporites, only known from megaspores, is thought to represent one of the oldest seed plants on earth (Marshall and Hemsley 2003). Among the Escuminac invertebrates, the scorpion Petaloscorpio (Fig. 4c) and the millipede Zanclodesmus (Fig. 4b) are considered to be some of the earliest continental arthropods (Jeram 1996; Wilson et al. 2005).
17 Most major groups of Devon-ian aquatic vertebrates are represented in the Miguasha biota with the noticeable exception of chondrichthyans and tetrapods (Fig. 7). The anaspid or anaspid-like Endeiolepis and Euphanerops (Fig. 8j) are the last survivors of a group that originated in the Silurian (Janvier 1996a, b), and are likely closely related to living lampreys (Gess et al. 2006); however, these two genera are occasionally referred to simply as euphaneropids because of their questionable relationships to either anaspids or lampreys (Janvier and Arsenault 2007). The actinopterygian Cheirolepis (Fig. 8h) is among the most basal rayfinned fishes (Cloutier and Arratia 2004), the most diverse group of living vertebrates. Sarcopterygians (Fig. 8a–b, d–g, i), or lobe-finned fishes, are well represented at Miguasha; the actinistian Miguashaia (Fig. 8g) is a basal coelacanth (Cloutier 1991a, 1996b; Friedman and Coates 2006), a group that today includes only two species (Latimeria chalumnae and L. menadoensis). The osteolepiform Eusthenopteron foordi (Fig. 8a) is one of the best-known Miguasha fossils and for nearly one hundred years was considered transitional between fishes and the first tetrapods. Because of its exceptional state of preservation, anatomical completeness, and the extensive lifelong study by Erik Jarvik, Eusthenopteron foordi could to some extent be considered a ‘model organism’. Finally, Elpistostege watsoni is now thought to be one of the fish species closest to tetrapods (Schultze 1996). The anatomy of Elpistostege is poorly known, based so far on only four fragments (Fig. 8e), but distinctive cranial features, such as the presence of frontal bones, are similar to those of early tetrapods. Daeschler et al. (2006) considered that either Elpistostege or Tiktaalik from the Canadian Arctic constitute a sister-group to tetrapods.
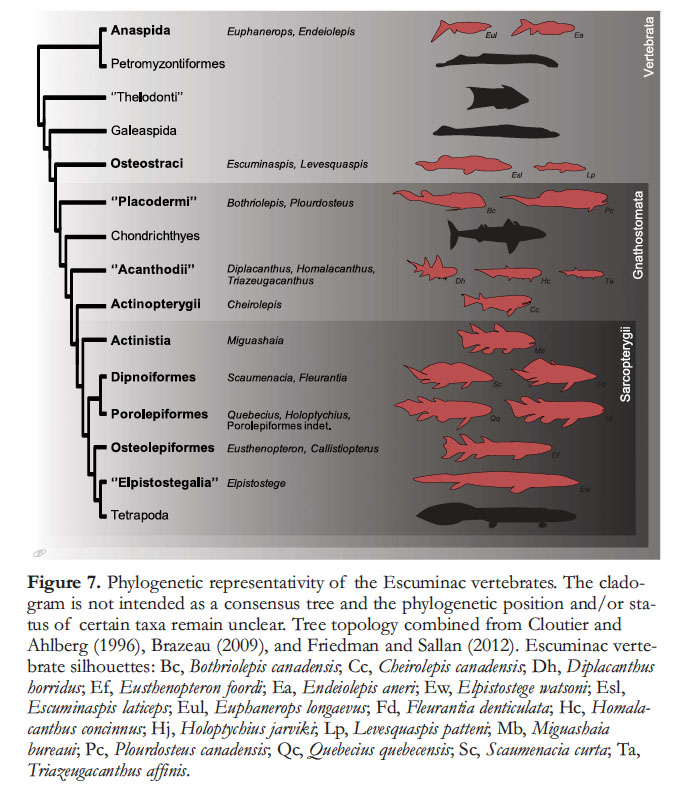 Display large image of Figure 7
Display large image of Figure 7 Display large image of Figure 8
Display large image of Figure 818 Because of their significant evolutionary status, some of the Miguasha fish species have been systematically included in the majority of phylogenetic analyses published over the past 20 years: Euphanerops in lower vertebrate phylogenies (Donoghue et al. 2000; Gess et al. 2006; Sansom et al. 2010); Triazeugacanthus and Homalacan-thus in basal gnathostome phylogenies (Brazeau 2009; Davis et al. 2012); Cheirolepis in actinopterygian and osteichthyan phylogenies (Cloutier and Arratia 2004); Miguashaia in actinistian, sarcopterygian and osteichthyan phylogenies (Cloutier 1991b; Forey 1998; Zhu et al. 2009); Scaumenacia in dipnoan phylogenies (Lloyd et al. 2012); Eusthenopteron in osteolepiform, sarcopterygian, and early tetrapod phylogenies (Cloutier and Ahlberg 1996; Ahlberg and Johanson 1998); and Elpistostege in early tetrapod phylogenies (Daeschler et al. 2006; Ahlberg et al. 2008).
Paleoecological Significance of the Miguasha Biota
19 Over the years, an impressive number of paleoecological interactions among the various components of the Devon-ian Miguasha biota have been reconstructed, providing the trophic structure of one of the oldest diverse vertebrate assemblages. Paleobiological information for numerous Escuminac species has been recovered based on the presence of bite marks, ingested prey, regurgitates, cololites (feces still located in intestinal tract), and coprolites (Fig. 9).
 Display large image of Figure 9
Display large image of Figure 920 The first case of predation reported was an acanthodian, Homalacanthus, found in the digestive tract of the osteolepiform Eusthenopteron (Arsenault 1982). Subsequently, cannibalism was reported for Cheirolepis (Arratia and Cloutier 1996) and recently observed in Eusthenopteron (Cloutier et al. 2011; Fig. 9b). In addition to such direct evidence of trophic level partitioning, abundant regurgitates and coprolites have been found with bony inclusions (McAllister 1996). McAllister (1996) identified prey items in coprolites that included acanthodian scales, spines and shoulder elements, actinopterygian scales and bony elements, sarcopterygian scales, and conchostracan valves. Predation scars (bite marks) on a Miguasha fish species are reported here for the first time. A series of small circular bite marks, most likely inflicted by an osteichthyan, have been found on the surface of cephalic shield bones of Bothriolepis canadensis (Fig. 9c).
21 The overall paleoecology and food web of the aquatic Miguasha biota has been inferred from the gross morphology of the organisms and comparisons with closely related forms (Janvier 1996a; Cloutier et al. 2011), as well as from direct evidence of predation. Bacteria and other micro-organisms constitute the decomposers of the Miguasha biota. Evidence of bacterial activity is suggested by the abundance of concretions (see Fig. 1b) containing organic remains (e.g. partial to complete fishes; El Albani et al. 2002); these concretions are thought to have formed as the result of bacterial metabolism. Furthermore, bacterial mats (here reported for the first time) have been observed to cover soft body tissues of Miguasha fishes (Fig. 10c). The paucity of trace fossils and the absence of bioturbation suggest that the bottom was not a particularly hospitable environment. Conchostracans (Asmusia membranacea) were the primary consumers of the Miguasha biota. Conchostracans are non-selective algal and detrital feeders (Orr and Briggs 1999), and their valves have been found in the digestive tracts of Bothriolepis, Homalacanthus, and Scaumenacia (Cloutier 1996a; Cloutier et al. 2011). The anaspid Euphanerops and Endeiolepis were most likely microphagous bottom feeders, based on the presence of sediment in a structure interpreted as their stomach (Stensiö 1939; Janvier 1996a; Janvier and Arsenault 2007). The relatively flattened morphology and dorsal eye position of the osteostracan Escuminaspis and Levesquaspis, and the placoderm Bothriolepis, imply that they were also bottom dwellers. Escuminaspis and Levesquaspis were detritivores, most likely consuming both bottom detritus and particles and organisms suspended in the water column, as suggested for Devonian osteostracans from Russia (Moloshnikov 2008). Bothriolepis, Plourdosteus and the dipnoans are considered benthivores (Moloshnikov 2008). The four Miguasha acanthodian species were likely planktivores, as suggested for anatomically similar acanthodians from Scotland (Trewin 1986). Owing to their abundance, their relative small size and their common occurrence in both coprolites (McAllister 1996) and digestive tracts of predators, Homalacanthus and to a lesser extent Triazeugacanthus contributed as forage fish in the aquatic ecosystem. The actinopterygian Cheirolepis was a small predator, whereas large predators include the sarcopterygian Miguashaia, Holoptychius, Quebecius, the porolepiform indet., Eusthenopteron, and Elpistostege. Among predators, Cheirolepis (Arratia and Cloutier 1996) and Eusthenopteron (Arsenault 1982; Cloutier 1996c; Cloutier et al. 2011) have been found with ingested prey, which includes Homalacanthus, Triazeugacanthus, Cheirolepis, and Eusthenopteron. Based on numerous observations of ingested prey, at least a five-level food chain can be inferred (Fig. 9a); from bottom to top: the conchostracan Asmusia, Homalacanthus, small Cheirolepis, large Cheirolepis, and Eusthenopteron (Cloutier 2009). The trophic network is still incomplete, but is under active study (M. Chevrinais and R. Cloutier).
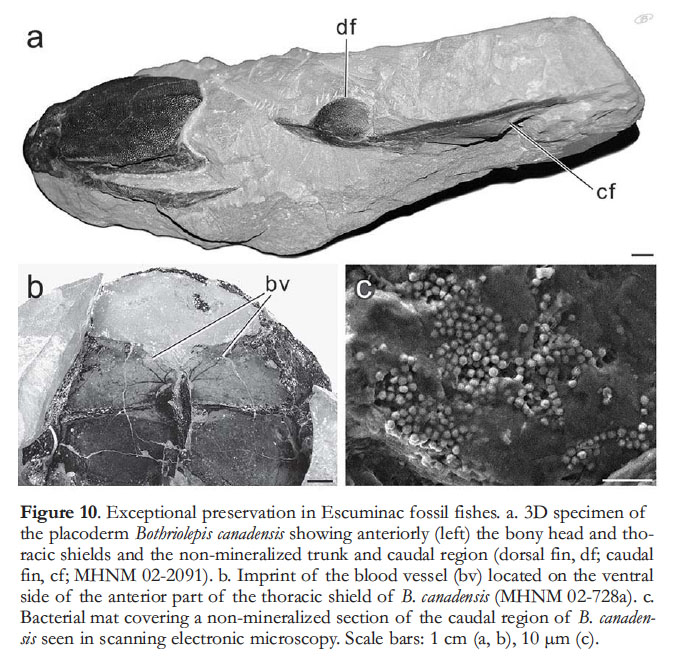 Display large image of Figure 10
Display large image of Figure 1022 Exceptional preservation allows studies on specific or group behaviour. Evidence of gregarious behaviour has been suggested for Both-riolepis (Fig. 4e), Triazeugacanthus and Scaumenacia (Cloutier et al. 2011). It is likely that the gregarious behaviour of these species is associated with particular periods in their respective life cycles, because groups are generally composed of similar-sized individuals or age classes (Parent and Cloutier 1996; Cloutier et al. 2011). Gregarious behaviour contributes to the high abundance of monospecific assemblages associated with different horizons in the formation.
23 From the base to the top of the Escuminac Formation, a predominant fish assemblage composed of three species (B. canadensis, S. curta and E. foordi) persists, while the presence of the remaining species fluctuates among the regressive and transgressive phases of the different stratigraphic sequences (Cloutier et al. 2011). This predominant assemblage might well represent a case of paleoecological stasis if we manage to more precisely establish the timespan encompassed by the complete stratigraphic sequence.
PALEOENVIRONMENT OF THE ESCUMINAC FORMATION
24 The depositional environment of the Escuminac Formation has been variously considered as lacustrine, estuarine, coastal marine or marine. An estuarine interpretation best accommodates the different lines of evidence provided by the fauna (Schultze and Cloutier 1996), the palynofacies (Cloutier et al. 1996), the trace fossil assemblage (Maples 1996), the sedimentological and stratigraphic setting of the formation (Hesse and Sawh 1992; Cloutier et al. 2011), and the geochemistry of the sedimentary rocks and bones (Schmitz et al. 1991; Vézina 1991; El Albani et al. 2002; Matton et al. 2012).
25 Over the past 15 years, we have accumulated evidence that the Miguasha biota inhabited an estuarine paleoenvironment, starting with the discovery of acritarchs in association with a large part of the fish assemblage (Cloutier et al. 1996). Furthermore, characterization of the organic matter by kerogen pyrolysis has revealed a predominance of Type II organic matter, which suggests a mixing from continental and marine sources (El Albani et al. 2002). Geochemical analyses of both sediment and bony elements from the Escuminac Formation also suggest a transitional environment. The strontium isotope ratio of Escuminac bony fragments reveals a Frasnian seawater signature, suggesting a slight diagenetic contamination, although fitting within the transitional range (Matton et al. 2012). Some facies contain evidence of tidal influence, such as thin rhythmites showing daily and lunar cycles (Cloutier et al. 2011). These were originally interpreted as lacustrine varves. Thinning of the rhythmites is associated with neap tides, whereas a thickening is associated with spring tides. Such rapid cyclic deposition, characteristic of the lower part of the Escuminac Formation, facilitated rapid burial of fish carcasses and allowed for the exceptional preservation typical of this facies. Tidal rhythmites provide a perfect host for Konservat– and Konzentrat–Lagerstätte horizons within the Escuminac Formation.
26 Five transgressive – regressive sequences have been recognized in the Escuminac Formation; these sequences are consistent with an inner wave-dominated estuary showing a shift towards continentalization (Cloutier et al. 2011). Throughout the formation, changes in richness, abundance and composition of the fish assemblages show that the transgressive phases are more diverse and better structured in terms of species composition than the regressive phases (Cloutier et al. 2011). In addition, rare and sporadic taxa, such as Levesquaspis, Plourdosteus, Miguashaia and Elpistostege, are found solely in the transgressive phases, whereas the most abundant species (Fig. 6) are found in both transgressive and regressive phases (Cloutier et al. 2011).
KONSERVAT– AND KONZENTRAT–LAGERSTÄTTEN
27 Fossils encountered in the Escuminac Formation show a wide range of preservational modes (Parent and Cloutier 1996; Cloutier et al. 2011), from specimens with no apparent sign of decay down to isolated bony elements. Contrary to popular perception, not all levels qualify as Lagerstätte horizons. Where exceptional quality of preservation predominates, the term Konservat–Lagerstätte can be applied; where diversity and abundance are outstanding, the horizon represents a Konzentrat–Lagerstätte.
28 The exceptional condition of preservation is indicated by the great number of fish specimens for which completely articulated specimens were found, such as multi-element skeletons of osteostracans and acanthodians (Fig. 8k–n). Although most specimens are compressed laterally, three-dimensional preservation is fairly common (Figs. 4d–e, 8a–c, e, 10a; Parent and Cloutier 1996). Not only is the external morphology well preserved, but detailed bone histology is also present either as hard tissues (e.g. enamel, dentine, cellular bone, cartilage) or as cell spaces (e.g. osteocytes, chondrocytes); examples include cartilages in Euphanerops (Janvier and Arsenault 2002), bone tissues in Bothriolepis (Downs and Donoghue 2009) and Eusthenopteron (Laurin et al. 2007; Zylberberg et al. 2010), and dental tissues of Scaumenacia (Thomson 1972; Smith et al. 1987) and Eusthenopteron (Schultze 1969). Recent investigations have focused on exceptional cases of fossilization where preservation of soft tissues such as digestive tracts, stomachs, spiral intestines, lungs, gill filaments, blood vessels (Fig. 10b) or muscles is involved (Arsenault et al. 2004; Janvier et al. 2006; Janvier et al. 2007; Cloutier 2009; Janvier and Arsenault 2009; Arsenault and Janvier 2010). In addition, preliminary studies on nonmineralized parts of the Bothriolepis body suggest that ‘skin preservation’ results from the presence of a bacterial mat outlining the surface of the carcass (Fig. 10a, c); this phenomenon is known as pseudomorphing (Briggs 2003).
29 Fossilized ontogenies are well represented in the Escuminac Formation. Exceptionally preserved larval and juvenile specimens (as small as 6 mm in total length) have been identified for 14 out of the 20 species of fishes (Cloutier et al. 2009), providing the opportunity to study growth and developmental change (e.g. Fig. 11a, f–g). One of the best examples of a fossilized growth series is that of the placoderm Bothriolepis, where specimens as small as 5 mm long to approximately 22 cm of shield length are known (Cloutier 2010; Fig. 11a). With such material, size and shape changes can be studied (Thomson and Hahn 1968; Schultze 1984; Werdelin and Long 1986; Cloutier 1997; Cloutier et al. 2009), as well as the process of ossification of some of these species (Cote et al. 2002; Cloutier 2010; Béchard and Cloutier 2011). These data can be compared among fossil and living taxa in order to study the evolution of development (Cote et al. 2002; Cloutier et al. 2009; Cloutier 2010).
 Display large image of Figure 11
Display large image of Figure 1130 Concentration of fossils is not limited to fish fragments, but extends to complete specimens. Konservat–and Konzentrat–Lagerstätte horizons occur throughout the Escuminac Formation. Certain horizons are best considered as Konzentrat–Lagerstätten because of their richness and abundance. One of the most outstanding horizons is bed 8 (see lower * in Fig. 3d; Fig. 11e), which includes 11 species. Many years of systematic collecting in bed 8 (Fig. 1d) provided the opportunity to create a GIS (geographical information system) plot in which more than 700 specimens are mapped within a section of a bed measuring approximately 25 m2 in area and 35 cm in thickness. A few years ago, a new Konzentrat– and Konservat–Lagerstätte (see upper * in Fig. 3d; Fig. 11b) was discovered, most likely corresponding to a fish nursery or an effective juvenile habitat (Béchard and Cloutier 2011). Over 1000 larval and juvenile specimens belonging to at least five species were found (Fig. 11b–d); numerous taphonomic, paleoecological, morphological and developmental aspects of this occurrence are currently under investigation at the Université du Québec à Rimouski.
CONCLUSION
31 Recognition of the Miguasha fossil locality as an exemplar of the Devon-ian ‘Age of Fishes’ and its designation as a UNESCO World Heritage Site (Cloutier and Lelièvre 1998) is based on: (1) its faunal representativity of major groups of sarcopterygians, (2) the representativity of vertebrate evolutionary events, (3) the floristic and faunal representativity of aquatic and continental assemblages, (4) the paleo-biological representativity (e.g. presence of ingested prey, presence of fossilized ontogenies), (5) the quality of preservation in terms of anatomical completeness, (6) the quality of preservation in terms of exceptional fossilization, and (7) the abundance of specimens. The Devonian Miguasha biota stands as a primary Fossil–Lagerstätte, a true time capsule in the early history of vertebrates.
Over the past 30 years I have had the privilege to collaborate on various aspects of the Miguasha biota with many colleagues, and I would like to especially thank G. Arratia, I. Béchard, A.-M. Candilier, F. Charest, M. Chevrinais, A. El Albani, J. Kerr, J. Leblanc, O. Matton, N. Parent, D. Potvin-Leduc, J.-N. Proust, H.-P. Schultze, R. Stevenson, and B. Tessier. I. Béchard helped with the preparation of the figures. I. Béchard, C. Bureau, M. Chevrinais, and J. Kerr provided some of the photographs. I. Béchard, M. Chevrinais, P. Janvier, D. Rudkin, and H.-P. Schultze provided comments on earlier versions of the manuscript. Research carried out on the Miguasha biota has been financed by the Research Chair in Pale-ontology and Evolutionary Biology (UQAR-SEPAQ; Power Corporation Inc.), Natural Sciences and Engineering Research Council (NSERC Discovery Grant), the Parc national de Miguasha (SEPAQ), Ministère de l’Éducation, Loisir et Sport du Québec (Chantier 3 Grant), and the CNRS (USTL1-Lille and Geosciences Rennes).
