Investigations of Burial Diagenesis in Carbonate Hydrocarbon Reservoir Rocks
Hans G. MachelDepartment of Earth and Atmospheric Sciences, University of Alberta, Edmonton, AB, Canada, T6G 2E3.
hans.machel@ualberta.ca
SUMMARY
Investigations of burial diagenesis are instrumental for hydrocarbon exploration and exploitation. A proper investigation of diagenesis, with the aim to assist in exploration for and exploitation of hydrocarbons, should follow the "6 -Step Process". Step 1 : facies analysis (including establishing the primary porosity and permeability distributions, and the "primary aquastratigraphy" - a term newly defined in this article); Step 2 : petrographic analyses (paragenetic sequence, mapping amounts and spatial distribution of diagenetic phases); Step 3 : geochemical analyses (isotopes, trace elements, fluid inclusions, etc.); Step 4 : burial history and paleohydrology; Step 5: integration with extant data (especially petrophysical data), if available, and Step 6 : modeling (not necessary, but desirable in at least some cases). Diagenesis, at any depth from near-zero to several kilometres, is governed by various intrinsic and extrinsic factors that include thermodynamic and kinetic constraints, as well as microstructural factors. These factors govern diagenetic processes such as cementation, dissolution, compaction, recrystallization, replacement, and sulfate-hydrocarbon redox-reactions. Cementation, dissolution, and dolomitization require significant flow of groundwater (of whatever type and/or salinity, ranging from fresh to hypersaline), driven by an externally imposed hydraulic gradient. Other processes, such as stylolitization and thermochemical sulfate reduction, commonly take place without significant groundwater flow in hydrologically stagnant systems that are geochemically closed. Two effects of diagenesis that are especially important for hydrocarbon reservoirs are enhancement and/or reduction of porosity and permeability. However, these rock properties can also remain essentially unchanged through diagenesis at depths from near-zero to several kilometres. In extreme cases, an aquifer or hydrocarbon reservoir rock can have highly enhanced porosity and permeability because of extensive mineral dissolution, or it can be plugged up by extensive mineral precipitation.SUMMAIRE
Les études de diagenèse d'enfouissement sont des instruments essentiels dans les domaines de l'exploration et de l'exploitation des hydrocarbures. Une étude de la diagenèse ayant comme objectif de contribuer à l'exploration et l'exploitation des hydrocarbures devrait suivre le processus suivant en six étapes : Étape1) L'analyse des faciès (comportant la mesure de la distribution de la porosité et de la perméabilité initiales, ainsi que de l'" aqua-stratigraphie " - terme redéfini dans le présent article; Étape 2) Les analyses pétrographiques (séquence paragénétique, cartographie de la répartition volumique et spatiale des différentes phases diagénétiques); Étape 3) Les analyses géochimiques (isotopiques, d'éléments traces, des inclusions fluides, etc.); Étape 4) L'historique d'enfouissement et la paléohydrologie; Étape 5) L'intégration avec les données existantes (particulièrement les données pétrophysiques), et Étape 6) La modélisation (pas nécessaire mais utile dans certains cas). Qu'il s'agisse de très faibles profondeurs ou de profondeurs de plusieurs kilomètres, la diagenèse est un phénomène qui est déterminé par des facteurs intrinsèques et extrinsèques, incluant des facteurs thermodynamiques et cinétiques, ainsi que microstructuraux. Ces facteurs déterminent des processus diagénétiques comme la cimentation, la dissolution, la compaction, la recristallisation, la substitution, ainsi que les réactions d'oxydoréduction sulfate-hydrocarbures. La cimentation, la dissolution et la dolomitisation suppose la circulation de volumes considérables d'eaux souterraines (peu importe le type et ou la salinité, qu'elles soient douces ou hyper-salines), mobilisés par les gradients hydrauliques ambiants. D'autres processus comme la stylolitisation et la réduction thermochimique des sulfates, se produisent généralement sans apport substantiel en eau dans le contexte de systèmes hydrologiques stagnants et géochimiques clos. La bonification et ou la détérioration de la porosité et de la perméabilité sont deux des effets diagénétiques particulièrement importants dans la caractérisation des réservoirs d'hydrocarbures. Cependant, ces propriétés lithologiques peuvent demeurer presqu'inchangées par la diagenèse qu'elle se produise à des profondeurs faibles ou de plusieurs kilomètres. Dans les cas limites, un aquifère ou un réservoir d'hydrocarbures peut comporter des porosités et des perméabilités qui auront été grandement bonifiées par l'action d'une dissolution minérale importante, ou voir leurs pores colmatés par l'action d'une précipitation minérale importante.
INTRODUCTION
1 Exploration for hydrocarbons is, first and foremost, the search for rocks with high porosity and permeability. These two basic petrophysical properties control almost all other hydrocarbon reservoir properties. Most importantly, the higher the porosity and permeability, the higher the chances for economically viable oil and gas storage, and for high flow rates during exploitation.
2 Porosity and permeability are originally controlled by sedimentary conditions at the time of deposition, and then modified by diagenetic alteration. In rocks that have not been buried deeply and/or that are relatively young, the reservoir properties are governed largely by depositional and facies parameters, such as water energy, grain size distribution, grain packing density, sorting and rounding, reef framework, etc. Overall, however, such reservoir rocks are in a minority. Most sediments and sedimentary rocks have been buried to several hundreds or thousands of meters for millions of years, with or without subsequent uplift. Commonly, the reservoir properties of such rocks are controlled by diagenesis because, with few exceptions, the effects of diagenesis increase with burial depth and time. Hence, investigations of diagenesis are instrumental for hydrocarbon exploration and exploitation, especially in deep basins.
3 Regardless of depth, investigations of diagenesis must take into account the conditions during and right after deposition, which determine the types and amounts of primary porosity and the chemistry of the pore water that may or may not have been buried with the sediments/rocks. These conditions are encompassed by the concept of "primary aquastratigraphy", as defined further below. The focus of this article, however, is on burial diagenesis, and especially on carbonates that may act as petroleum reservoir rocks. Fortunately, many if not most of the phenomena covered herein are valid for and/or applicable to both carbonates and clastics. Moreover, carbonate diagenesis cannot be treated fully without considering other rock types because of the chemical intercommunication of all rock types via hydrologic flow.
4 This article is geared primarily to individuals, students as well as professionals, who are relatively new to carbonate diagenesis and petroleum reservoir rocks. It provides an overview of the most relevant investigative methods and provides a ‘ cookbook recipe' for investigations of diagenesis (the so-called "6 - Step Process"). The ultimate goal of this paper is to aid reservoir characterization, especially the present porosity and permeability distribution that can be used in the development of hydrocarbon reservoirs by production engineers, as well as in exploration in terms of prediction of porosity and permeability.
METHODS OF INVESTIGATION
5 The various diagenetic processes and their effects on porosity and permeability can be investigated and/or characterized by a variety of methods that fall into two major groups (Fig. 1). The present group is used to establish present porosity and permeability, whereas the past group is used to establish past porosity and permeability and/or the evolution of these parameters through time.
6 There are two important aspects to the present group. First, this group covers all orders of magnitude from satellite images to microprobe spot analyses, at least in principle. In the petroleum industry, data from seismic, outcrop and/or core are almost always available, as are data from thin sections and the various microscopic methods of investigation. Hence, these are the standard tools by which the spatial distribution and sizes of pore spaces are determined. However, the various types of remote sensing and/or GIS have limited, albeit in some cases highly useful, applicability. For example, Harris and Kowalik (1994) have shown how the present distributions of porosity and permeability can be estimated from a series of satellite images of areas that are recent analogs to certain types of sedimentary hydrocarbon traps. This type of analysis can only be used when suitable satellite images are available, and when the sedimentary environment of interest in a given hydrocarbon play has a recent analog that can be imaged from outer space. Similarly, other types of remote sensing, such as aeromagnetics, gravity, etc., have limited applicability.
7 The present group of methods is further divided into two subgroups, i.e., those methods that are qualitative and/or semiquantitative, and those that are quantitative. The first subgroup, to the left of the vertical dotted line in Figure 1, characterizes the spatial distribution and sizes of porosity and permeability, whereas the second subgroup determines absolute amounts. Microbeam techniques (imaging, trace elements, isotopes) straddle this line, and thin section petrography and image analysis connect the two subgroups. Thin section, SEM, and microprobe images can be used to quantify the amounts of diagenetic phases and porosity (e.g., Weidlich et al., 1993; Fagerstrom and Weidlich, 1999). Petrophysical methods of investigation, i.e., mercury injection capillary pressure measurements, as well as direct porosimetry and permeability measurements on whole core or core plug samples can also be used effectively in the context of diagenetic investigations because they are quantitative (e.g., Luo and Machel, 1995; Lucia, 2000).
Figure 1. Methods of investigation of present and past porosity and permeability.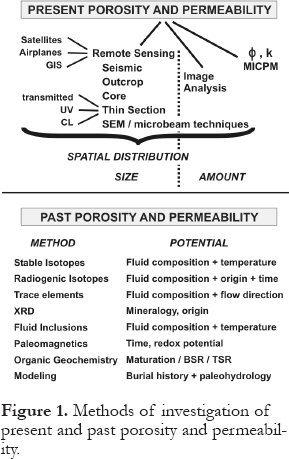
Display large image of Figure 1
8 The past group of methods (Fig. 1) is composed of most of the standard tools of diagenetic investigation on microscopic and submicroscopic scales, including inorganic and organic geochemistry, as well as various types of modeling (burial history, paleohydrology, etc.). One should not expect unequivocal answers from any method. Rather, each method has the potential to reveal certain aspects of a rock's past, or its evolution through time. Unfortunately, some methods may fail to work in a given study. For example, fluid inclusion analysis may fail to provide reliable maximum burial temperatures because the inclusions are too small, or modeling of paleofluid flow may fail because the paleopermeability cannot be estimated with reasonable accuracy.
DIAGENETIC SETTINGS
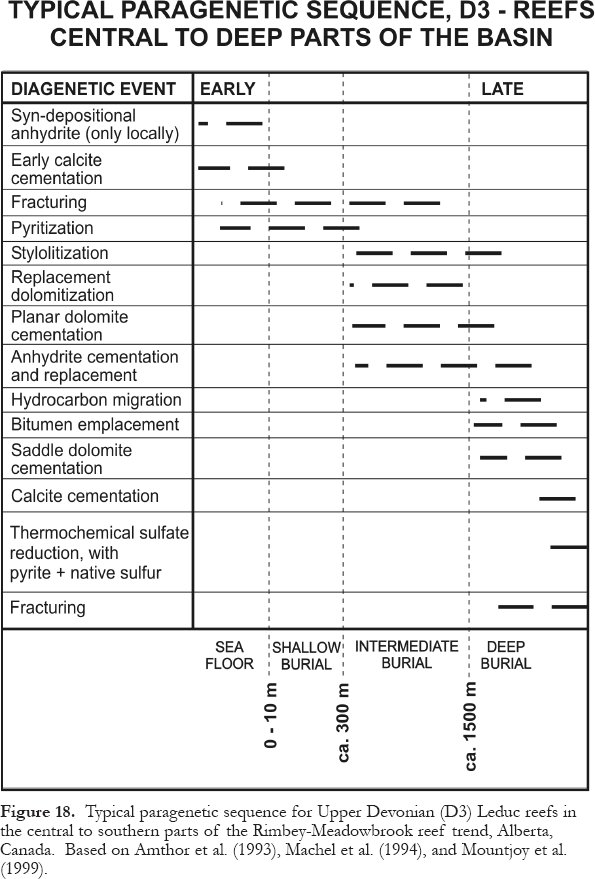
9 The realm of diagenesis comprises all mineralogical, physical, and chemical changes of sedimentary deposits between the time of ultimate deposition and the onset of metamorphism (green-schist facies). Diagenetic settings are commonly divided into "shallow," "intermediate," and "deep" burial types; yet in many case studies these terms are ill-defined and hydrological/hydrogeochemical criteria are commonly ignored (a noteworthy exception is Galloway and Hobday, 1983). For these reasons, Machel (1999) proposed a new classification that is based on a critical synopsis of previous classifications and is applicable to all rock types (Fig. 2). This classification uses mineralogic, geochemical, and hydrogeologic criteria from clastic and carbonate rocks, the occurrence of hydrocarbons, and fractures. It differentiates near-surface, shallow-, intermediate-and deep-burial diagenetic settings, hydrocarbon-contaminated plumes, and fractures.
Near-Surface Diagenetic Settings
10 Near-surface diagenetic settings are those within a few metres of surface, where the pore fluids are essentially unaltered and of meteoric, brackish, marine, or evaporitic origin. Marine diagenetic settings, which can be subdivided into various subsettings (e.g., James and Choquette, 1990), are included here because of the significant marine diagenesis many carbonates experience before they are buried beyond the reach of surface-derived groundwaters. The hydrologic drives in near-surface settings vary from place to place; they include wind action, wave action, and tidal pumping in marine settings; density-driven reflux in evaporitic settings; and gravity drive in vadose/meteoric settings.
Shallow-Burial Diagenetic Settings
11 Shallow-burial diagenetic settings (also called shallow subsurface settings or environments) are similar in many respects, including hydrogeochemistry, to near-surface diagenetic settings. Important differences include physical compaction and hydrologic conditions that may vary with the (paleo-) geographic setting. For example, much if not most, pore-water flow at very shallow depths is facilitated by tidal pumping in marine settings, giving way to compaction with increasing depth. In coastal mixing zones, pore water throughput is facilitated by the convergence of two forces, gravity (topography) drive from meteoric water and density drive from seawater. In evaporitic settings, most fluid throughput is via density-driven reflux. Subaerially exposed settings are generally governed by gravity drive in local groundwater flow systems that respond to surface conditions such as weather, seasons, and latitude; all have local groundwater flow systems. An important exception, from a hydrogeochemical point of view, is where local and regional groundwater discharge areas merge, and where deep(er) groundwaters mix with shallow groundwaters, providing for special hydrogeochemical settings.
Intermediate- and Deep-Burial Diagenetic Settings
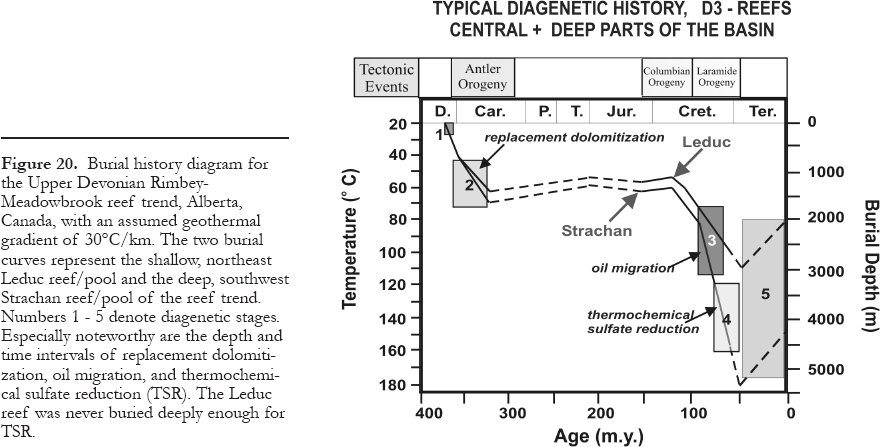
12 The lower limit of intermediate-burial diagenetic settings, and thereby the upper limit of deep-burial diagenetic settings, is defined as the top of the liquid oil window in hydrocarbon source rocks. This boundary is useful because the introduction of oil into the pore spaces commonly arrests mineral diagenesis. Unfortunately, the depth to the top of the oil window varies widely, i.e., between about 1500 and 4000 m depending on kerogen type and geothermal history, with a distribution maximum of about 2000 to 3000 m (Hunt, 1996). The latter depth interval is herewith taken as the bottom of intermediate-burial diagenetic settings. Deep-burial diagenetic settings merge into the metamorphic realm at temperatures around 200°C and commensurate depths and pressures, which depend on the geothermal gradient, e.g., about 6 km and 150 MPa (1.5 kbars) at 30°C/km, or about 9 km and 225 MPa(2.25 kbars) at 20°C/km (Winkler, 1979).
Figure 2. Classification of diagenetic settings on the basis of mineralogy, petroleum, hydrogeochemistry, and hydrogeology. For illustrative simplicity, the geologic section is assumed to be isotropic and homogeneous, with idealized groundwater flow lines. The hydrocarbon-contaminated plume is slightly deflected by the local and regional groundwater flow systems. The depth limits separating the burial diagenetic settings are approximate and based on geologic phenomena that are easily recognizable. Near-surface settings may be meteoric, brackish, marine, or hypersaline. Adapted from Machel (1999).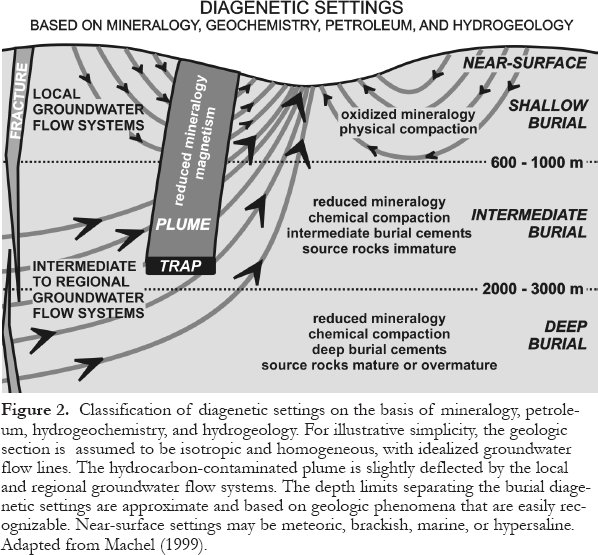
Display large image of Figure 2
13 The vast majority of sedimentary rocks spend most of their existence in the intermediate- and deep-burial realms, where porosity and permeability are commonly altered significantly, and where hydrocarbon maturation, migration, and most trapping occur. The associated groundwaters (also called burial fluids, formation fluids, subsurface brines, etc.) have compositions that are largely or entirely controlled by rockwater interactions.
14 In immature basins, the predominant hydrologic drive is compaction. In mature basins, intermediate- and deep-burial diagenetic settings are located within intermediate or regional ground-water flow systems (sensu Tóth, 1963; Lovley and Chapelle, 1995). Rocks in intermediate-burial settings experience chemical compaction as well as subsurface cementation and dissolution.
Petroliferous (Hydrocarbon-Bearing) Plumes
15 Petroliferous (hydrocarbon-contaminated) plumes, which originate from leaking oil and gas traps, represent a special type of diagenetic setting that may crosscut all previously defined settings. The escaping hydrocarbons tend to form subvertical plumes of moderately to strongly reducing conditions. The plumes tend to facilitate diagenetic reactions that are absent, or at least much less pronounced, outside the plumes, such as the formation of magnetic mineral assemblages, of carbonate cements with distinctive cathodoluminescence and/or isotopic composition, soil gas anomalies, distinctive vegetation, and a number of other phenomena (Barker et al., 1991; Burton et al., 1993; Al-Shaieb et al., 1994; Machel, 1995; Tedesco, 1995; Schumacher and Abrams, 1996).
Fractures
16 Another special type of diagenetic setting is fractures, which may be restricted to one of the diagenetic settings defined above or may transgress two or more of them. In the first case, diagenesis within the fractures is generally similar to that within the adjacent pore network. In the latter case, diagenesis within the fractures may be significantly different from that in the adjacent wall rocks, depending on how fast the waters/fluids move through the fractures, and whether these waters/fluids are significantly warmer, colder, underpressured, or overpressured relative to those in the wall rocks.
Temporal Designations
17 A paragenetic sequence lists diagenetic processes in the chronological order that they affected the rocks under investigation. The various processes are commonly given temporal designations, such as "early" or "late". However, such temporal designations are only relative and generally meaningless hydrogeochemically. For example, a "late-diagenetic" 16th phase in a complicated series of 18 phases may have formed in a near-surface setting from meteoric water perhaps only a few tens to hundreds of thousands of years after deposition, which is not uncommon in Cenozoic carbonates (e.g., Schroeder and Purser, 1986). Alternatively, a relatively "early-diagenetic" 5th phase may have formed at a depth of nearly 3 km some 300 m.y. after deposition, which is common in Paleozoic carbonates where the truly early diagenetic phases, those that formed during shallow burial, are obliterated and/or rendered unrecognizable by pervasive recrystallization (e.g., Schroeder and Purser, 1986). Hence, temporal designations should be used only in conjunction with the diagenetic settings shown in Figure 2.
POROPERM REDUCTION, PRESERVATION, AND ENHANCEMENT
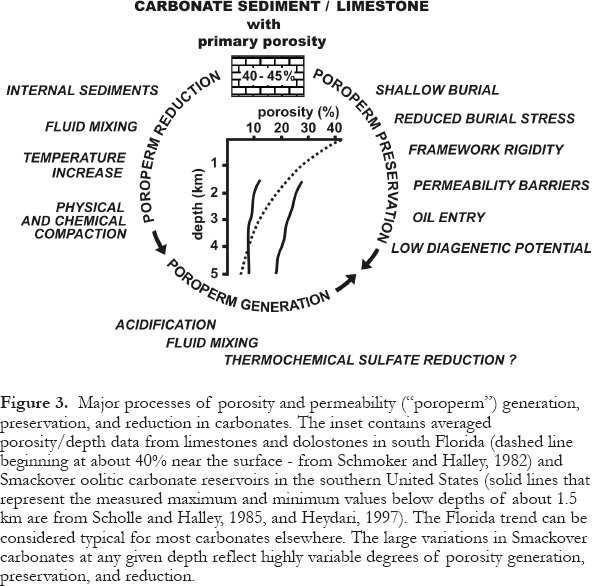
18 Porosity and permeability (poroperm) can be reduced, preserved, or enhanced through diagenesis. Figure 3 illustrates this phenomenon and the controlling factors for the well-studied Cenozoic limestones and dolostones from Florida and the Jurassic Smackover oolite reservoirs in the southern United States. Even though the trends are generally toward porosity reduction with depth, some Smackover rocks have much higher, others much lower, porosities than the average. Permeabilities are assumed to decrease with decreasing porosities. Although comparable permeability/ depth data sets are not available from these studies, they are available from studies of dolomitization. Commonly permeability increases along with porosity, and vice versa. This is documented through studies from the Late Devonian Grosmont Formation in eastern Alberta (Luo et al., 1994; Luo and Machel, 1995; Machel and Huebscher, 2000) and from the Cambrian-Ordovician Bonneterre Formation of Missouri, USA., which is host to one of the world's largest MVT-sulfide deposits (Woody et al., 1996). In both cases, there are significant positive correlations between porosity and permeability.
19 Based on this evidence, the porosity and permeability distribution in Cenozoic limestones and dolostones from Florida and the Jurassic Smackover rocks can be considered typical for many if not most carbonate rocks elsewhere. Their vertical distribution apparently can be highly variable, depending on the relative importance of the various processes involved. These processes begin syndepositionally and are governed by a number of intrinsic and extrinsic factors.
Intrinsic and Extrinsic Factors
20 Diagenetic processes that affect porosity and permeability in any type of sedimentary rock are cementation, dissolution, compaction, recrystallization, replacement (in carbonates, predominantly dolomitization), and sulfatehydrocarbon redox-reactions. To be volumetrically significant, cementation, dissolution, and dolomitization require significant flow of groundwater driven by an externally imposed hydraulic gradient because of the relatively low aqueous solubilities of almost all minerals. Other processes can and commonly do take place without significant groundwater flow, or even in hydrologically stagnant and consequently geochemically "closed" systems. Physical compaction is intermediate between these two groups. Physical compaction is not stagnant, as it generates flow, but it does not require an externally imposed driving force other than sedimentary loading.
21 The above diagenetic processes are governed by several factors including increasing temperature and pressure with depth, chemical changes in the pore fluids, and various types of groundwater flow (e.g., Hanor, 1987, 1994). The rocks influence, and are affected by, these factors – an interplay that is commonly called "water-rock interaction".
22 As far as these factors are concerned, diagenesis of one rock type cannot be separated from the diagenesis of other rock types, especially in intermediate- to deep-burial settings, where intercommunication of all rock types by groundwater flow is common. Nowhere is this more obvious than in the case of subsurface dissolution. Many carbonates have secondary porosity from dissolution in diagenetic burial settings, and most processes that generate acids originate in, or are facilitated by, clastic and carbonaceous rocks (e.g., Giles and Marshall, 1986; Surdam et al., 1989).
Thermodynamic Constraints
23 Cementation and dissolution are governed mainly by the saturation state. In general, temperature, pressure, and composition of the groundwater(s) determine the saturation state(s) and, hence, precipitation and dissolution, either directly or indirectly in one or several ways.
- Pore fluids in sedimentary basins, if buried and not changed significantly through water-rock interaction or mixing, become supersaturated with respect to carbonates and sulfates with depth, even if the concentrations of the cations (calcium, magnesium, iron, etc.) and carbonate ions remain essentially unchanged. Conversely, ascending formation/ groundwaters that cool tend to become undersaturated with respect to carbonates and sulfates, with the potential to generate significant secondary porosity in carbonates and/or clastics that contain carbonate cements (e.g., Giles and deBoer, 1989). The reverse is true for silicates and most other mineral groups. This is because of the temperature-dependent retrograde and prograde solubility of carbonates and sulfates versus silicates and other mineral groups, respectively.
- The concentrations of the cations and carbonate ions in solution generally increase because of pressure solution and/or a variety of diagenetic reactions involving non-carbonate minerals, such as silicates and sulfates. A common result is precipitation of carbonates because of the common-ion effect (e.g., Hanor, 1987).
- An increase in acidity can take place in many ways, including meteoric water penetration, mixing of waters, carbon dioxide generation from maturation of organic matter, generation of organic acids, and formation of acids from clay mineral reactions (e.g., Giles and Marshall, 1986); it leads to carbonate dissolution.
- Mixing of groundwaters may lead to pronounced undersaturation or supersaturation with respect to carbonate minerals, depending on temperature, pressure, and fluid composition, particularly pCO2 of the mixed water. Mixing of shallow groundwaters at relatively low temperatures commonly results in under-saturation and dissolution, which is well known from karst terrains (e.g., Bonacci, 1987; James and Choquette, 1987; Smart et al., 1988) and from caves in coastal seawater-freshwater mixing zones (e.g., Machel and Mountjoy, 1990). However, mixing of fresh groundwater with brine in deep burial settings commonly leads to supersaturation and carbonate precipitation (Morse et al., 1997).
- Pressure decreases, with or withouta significant temperature decrease, almost invariably leads to carbonate supersaturation and precipitation (Morse et al., 1997), either because of reverse solubility, CO2 -degassing, or both.
24 Under the assumption that minerals and solutions attain local thermodynamic equilibrium along the flow path, the above processes can be quantified in computer simulations to predict the precipitation and dissolution of diagenetic minerals along the flow path in basin strata as groundwaters migrate along temperature and pressure gradients. Using this approach, rock porosity evolution can be calculated from relative amounts of mineral precipitation and dissolution (e.g., Lee, 1997; Morse et al., 1997). Permeability changes, however, can merely be inferred, as pore throat sizes and tortuosity cannot be predicted from thermodynamics.
Figure 3. Major processes of porosity and permeability ("poroperm") generation, preservation, and reduction in carbonates. The inset contains averaged porosity/depth data from limestones and dolostones in south Florida (dashed line beginning at about 40% near the surface - from Schmoker and Halley, 1982) and Smackover oolitic carbonate reservoirs in the southern United States (solid lines that represent the measured maximum and minimum values below depths of about 1.5 km are from Scholle and Halley, 1985, and Heydari, 1997). The Florida trend can be considered typical for most carbonates elsewhere. The large variations in Smackover carbonates at any given depth reflect highly variable degrees of porosity generation, preservation, and reduction.Diagenetic Potential and Kinetic Factors
25 Both carbonates and clastics have different diagenetic potentials during burial, i.e., in any diagenetic setting certain minerals or types of grains exist that dissolve, form, or recrystallize preferentially over others. Such phenomena are governed by textural and/or kinetic factors. One of the best known examples is that small grains dissolve more rapidly than large grains during recrystallization and/or Ostwald ripening (e.g., Folk, 1965; Morse and Casey, 1988). Another example of microstructural control is that aragonite with complex microstructures can dissolve more rapidly than thermodynamically less stable magnesian calcite (Walter, 1985). Kinetic factors can override thermodynamic constraints during precipitation, particularly in near-surface and shallow diagenetic settings. Examples include the well-known kinetic inhibition of dolomite formation from seawater, and the formation of ‘ protodolomite' rather than dolomite from evaporated groundwater or seawater (e.g., Machel and Mountjoy, 1986; Usdowski, 1994). Such kinetic problems are ignored in the remainder of this paper, however, under the assumption that most diagenetic settings have had enough time to adjust to a thermodynamic equilibrium mineral assemblage. This certainly is true for most intermediate- and deep-burial settings, where sudden incursions of "alien" formation fluids are relatively rare, and where flow rates are generally low, except in fracture systems.
26 Another important factor, especially in intermediate- and deep-burial settings, is that hydrocarbons commonly arrest diagenesis, either because they coat potentially reactive grain surfaces in oil-wet systems, or because the relative permeability to water is very low or zero in the case of high oil saturation in water-wet systems (e.g., Laudon, 1996). In both cases, ionic exchange through the aqueous phase (dissolution, precipitation), and hence diagenesis, is inhibited, except for local and volumetrically insignificant mineral redistribution by diffusion. However, some hydrocarbons may react with the rocks, as in the case of thermochemical sulfate reduction (discussed below).
Depositional Controls
27 In carbonates, much more so than in clastics, depositional environment and facies, especially cycle stacking patterns, play an important role in determining the porosity and permeability distribution and their subsequent evolution through burial diagenesis. A case in point is the Knox Group in Tennessee, which contains transgressive and regressice carbonate cycles (Montañez, 1997). The transgressive cycles, which originally were fairly porous and permeable, now consist of limestone and <10 to >50 vol% "late-diagenetic" dolomite having an average porosity of 8.2% and an average permeability of 70.2 md, which are interpreted to have formed during intermediate to deep burial. Petrographic data also show that these rocks were affected by extensive dissolution in intermediate- to deep-burial settings. However, the facies within regressive cycles are almost completely replaced by tight fine-crystalline dolomite that formed syn-depositionally from evaporated seawater. The transgressive carbonate cycles behaved like aquifers during later burial diagenesis, whereas the regressive cycles became aquitards almost syn-depositionally. Other examples of depositional control on diagenesis are reef and reef margin carbonates, which typically are much more porous and permeable than back-reef and deep fore-reef carbonates (e.g., Schneidermann and Harris, 1985; Schroeder and Purser, 1986). This commonly leads to a pronounced differentiation into "proto-aquifers" and "protoaquitards" at the time of deposition.
Near-Surface Settings
Marine Diagenesis
28 Most carbonate rocks originate as marine sediments having primary porosities around 40-45%. Hence, the first pore water to affect the carbonate sediments is seawater, and the diagenetic setting is near-surface marine, where the predominant hydrologic drives are wind/wave action and tidal pumping. The main effect is partial to extensive filling of primary pores with internal sediments and marine carbonate cements (James and Choquette, 1990), which leads to significant porosity reduction. In general, highly porous and permeable facies types affected by vigorous waves and tides tend to become more cemented than other facies types in hydrogeologically less vigorous settings. Recrystallization of metastable aragonite and magnesian calcites also occurs, but this generally has little effect on porosity/permeability development.
Coastal Mixing Zone Diagenesis
29 In coastal areas where seawater mixes with fresh water, the predominant diagenetic process affecting carbonate sediments and rocks is dissolution (Plummer, 1975). Groundwater fluxes in coastal mixing zones (also called "zones of dispersion") typically are quite high, and the effects are enhanced porosity and permeability, including caves, which are well known from many such settings. Other well known processes in coastal mixing zones are dolomite cementation and replacive dolomitization. However, the effects of these processes are volumetrically insignificant (less than 1 vol-%) in almost all cases (Machel and Mountjoy, 1990). Similarly, some coastal mixing zones form aragonite or calcite cements (Csoma and Goldstein, 2004), but mixing zone cementation is volumetrically negligible in most settings.
Meteoric Diagenesis
30 Meteoric waters in near-surface settings are almost always undersaturated with respect to carbonates because rain water is essentially devoid of earth alkali metals yet slightly acidic because of dissolved atmospheric CO2. Where the ground has a significant soil cover, pCO2 in the downward percolating rain water of the vadose zone can easily increase by two orders of magnitude because of extensive plant and microbial activity in the soil zone. This increase results in extensive dissolution in the upper few metres of burial, and the effects are increased porosity and permeability and/or physical weakness of the rocks. For example, in the Caribbean islands, many of which have a cover of Cenozoic carbonates that have not been buried by more than a few metres, the rocks are frail and crumbly because of a lack of near-surface cementation and/or subsequent meteoric dissolution (James and Choquette, 1984).
Hypersaline Diagenesis
31 The only volumetrically important and widespread diagenetic effect of ground-water flow on carbonates in near-surface evaporitic settings is reflux dolomitization. Typically, fresh groundwater and/or seawater that are evaporated to or beyond gypsum saturation flow seaward within the uppermost few metres of the carbonate sediments via density-drive. These fluids form finely crystalline and geochemically distinct dolomite, either as a replacement or as cement. The resulting dolostones typically are laterally extensive, yet thin, aquitards that may form effective seals for hydrocarbon trapping (e.g., Machel and Mountjoy, 1986; Moore and Wilde, 1986).
Shallow-Burial Settings
32 The four near-surface hydrogeochemical settings discussed above (marine, brackish, meteoric, and hypersaline) commonly persist into shallow-burial diagenetic settings, where physical compaction and, at greater depths, incipient chemical compaction, as well as a relatively minor amount of water-rock interaction occur. The major differences compared to near-surface settings are that deeper meteoric settings generally experience cementation and porosity/permeability loss rather than dissolution and porosity/permeability gain.
Physical (Mechanical) Compaction
33 With increasing burial, physical (mechanical) compaction leads to loss of water and porosity. Carbonate sediments can compact to about one-half of their original thickness under as little as 100 m of overburden by physical compaction alone (Choquette and James, 1987, 1990). Thereby, sedimentary particles and structures are modified and rearranged until a self-supporting framework is achieved at an average porosity of about 40%. Further burial will lead to grain deformation in the form of ductile (squeezing) or brittle (breakage) failure, followed by chemical compaction. Common textures generated by mechanical compaction include thinning of laminae between, and draping over, concretions; flattening of burrows, fenestrae, gas-escape structures, desiccation cracks, and skeletal or detrital grains; rotated grains; spalling of coated grains; swirling structures; telescoping (conversion of grain-poor to grain-supported textures); and planar to curviplanar grain contacts. In contrast to most other diagenetic processes and effects, the extent of physical compaction and concurrent porosity/permeability loss strongly depends on depositional setting and near-surface diagenesis. Specifically, shallow-water carbonates generally become strongly cemented and solidified in the near-surface marine setting, which inhibits later physical compaction. However, cementation is typically poor in deep-marine carbonates, such as chalks, which permit much more extensive physical compaction during burial (e.g., Choquette and James, 1987, 1990; Maliva and Dickson, 1992).
Intermediate- to Deep-Burial Settings
Chemical Compaction
34 Chemical compaction results from pressure solution that is caused by load or tectonic stress at grain contacts, because stress increases mineral solubility. In addition, clay minerals embedded in the carbonate matrix may enhance or reduce the carbonate solubility (depending on the types of clays and their composition). Other factors that influence the inception and course of pressure solution include the burial depth, pore water composition, mineralogy, and the presence or absence of organic matter (including oil and gas). Depending on the interaction of these factors, pressure solution in carbonates commonly begins perhaps within 200 to 300 m of burial, with the first microstylolites forming at minimum depths of ca. 500 m of burial (Lind, 1993; Fabricius, 2000). Where dolostones are associated with limestones, the latter tend to be more susceptible to pressure solution.
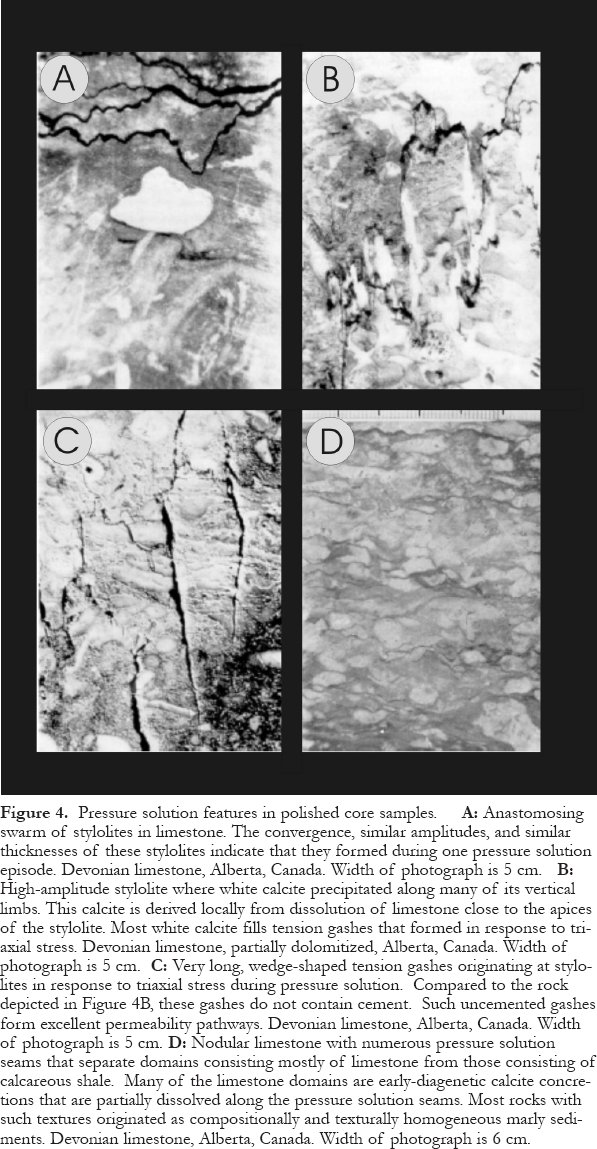
35 Pressure solution textures are diverse and include stylolites (one or several generations, typically with different amplitudes), other types of sutured seams, isolated or fitting nodules, nonsutured seams (Choquette and James, 1987, 1990), and pseudo-bedding that can resemble layering caused by sedimentary processes (Bathurst 1987; Munnecke et al., 2001; Fig. 4). Stylolites can be used to determine the number of burial episodes (number of generations) and the amount of compaction (calculations involve the number and amplitudes of stylolites: Bodou, 1976; Choquette and James, 1987, 1990; Ricken, 1987).
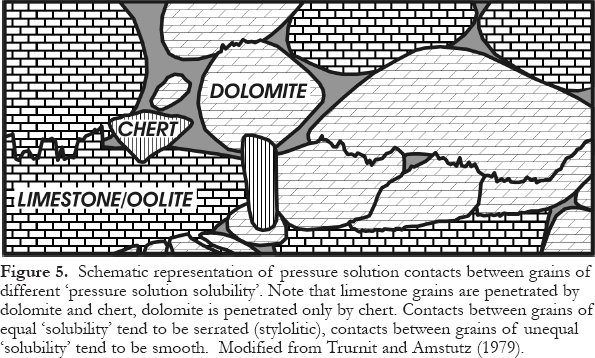
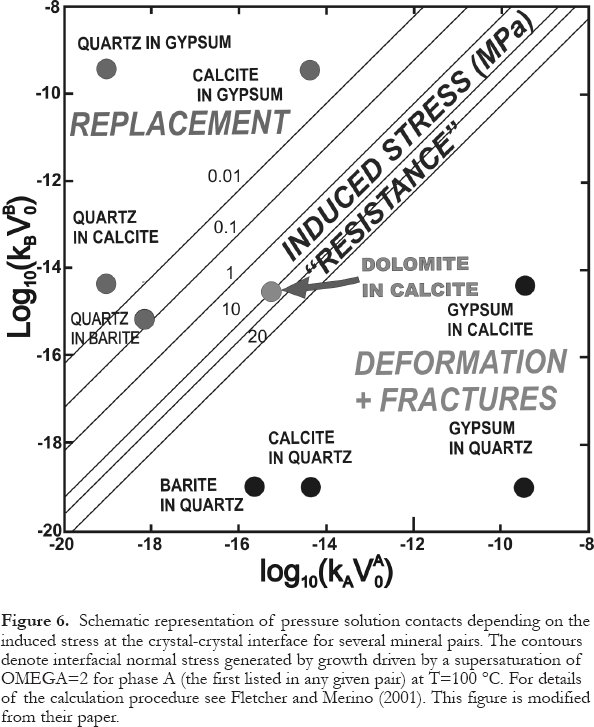
36 The type of interpenetration of adjacent grains during pressure solution depends on which minerals are juxtaposed. Where two grains are the same mineral, the pressure solution contact is sutured, as both grains dissolve to the same extent. However, the contacts are smooth where the mineralogy differs, as one mineral effectively grows at the expense of the other. For example, dolomite (mineral A) commonly grows into calcite (mineral B), and quartz commonly grows into calcite and/or dolomite, as illustrated in Figure 5. Such textures have been known for a long time, and the most common explanation given is that the solubility of mineral B is higher than that of mineral A at the contact point or surface. An alternative and more convincing explanation has been provided relatively recently by Fletcher and Merino (2001). These authors redefined and thereby expanded the principle of "force of crystallization" as "interfacial normal stress" (or "induced stress"). This stress is generated by growth driven by supersaturation of mineral A between crystals of minerals A and B, and the magnitude of this stress is dependent on the degree of supersaturation, temperature, as well as what the other mineral is. For example, at a temperature of 100 °C and a super-saturation of OMEGA = 2, quartz growing at the expense of calcite (quartz replacing calcite) generates a negligible stress, whereas calcite growing at the expense of quartz generates a huge stress of about 100 MPa (Fletcher and Merino, 2001). This effectively means that quartz penetrates calcite like a knife cutting into butter, as shown in Figure 5, where the pore water is supersaturated with respect to quartz yet saturated or undersaturated with respect to calcite. Conversely, calcite tends to deform or fracture adjacent quartz grains, if the pore water is supersaturated with respect to calcite yet saturated or undersaturated with respect to quartz. Both these scenarios are possible during pressure solution, depending on fluid composition. Figure 6 shows this and several other scenarios. Mineral pairs in the top left quadrant will show replacement textures, like "a knife cutting into butter", whereas the inverted mineral pairs in the bottom right quadrant will show deformation and/or brittle failure of the "weaker" mineral.
37 Chemical compaction begins during the latter phases of physical compaction and continues into the metamorphic realm (Choquette and James, 1987, 1990). Chemical compaction is most active in rocks that are being buried (as opposed to being stationary at some depth), and that have some porosity left to facilitate grain interpenetration and ionic movement from the points of dissolution into the pore fluids. The liberated ions move, via advection or diffusion, to areas of lower stress, either just adjacent to the area of dissolution or elsewhere in the pore system, where the dissolved material may reprecipitate, forming burial-diagenetic cements. Chemical compaction is known to result in thickness reductions of at least 25-35% in addition to the thickness losses caused by physical compaction. In the Smackover oolites, which were used to construct the porosity/depth curves in Figure 3, physical and chemical compaction combined account for a loss of 33% of the original 45% porosity (Heydari, 1997).
38 Chemical compaction generally is detrimental to reservoir rock characteristics and/or petroleum production. The major effects are porosity and permeability reduction as a result of the overall thickness reduction and reprecipitation of the material dissolved at grain contacts.
Figure 4. Pressure solution features in polished core samples. A: Anastomosing swarm of stylolites in limestone. The convergence, similar amplitudes, and similar thicknesses of these stylolites indicate that they formed during one pressure solution episode. Devonian limestone, Alberta, Canada. Width of photograph is 5 cm. B: High-amplitude stylolite where white calcite precipitated along many of its vertical limbs. This calcite is derived locally from dissolution of limestone close to the apices of the stylolite. Most white calcite fills tension gashes that formed in response to tri-axial stress. Devonian limestone, partially dolomitized, Alberta, Canada. Width of photograph is 5 cm. C: Very long, wedge-shaped tension gashes originating at stylolites in response to triaxial stress during pressure solution. Compared to the rock depicted in Figure 4B, these gashes do not contain cement. Such uncemented gashes form excellent permeability pathways. Devonian limestone, Alberta, Canada. Width of photograph is 5 cm. D: Nodular limestone with numerous pressure solution seams that separate domains consisting mostly of limestone from those consisting of calcareous shale. Many of the limestone domains are early-diagenetic calcite concretions that are partially dissolved along the pressure solution seams. Most rocks with such textures originated as compositionally and texturally homogeneous marly sediments. Devonian limestone, Alberta, Canada. Width of photograph is 6 cm.Cementation
39 Cementation is common in intermediate- and deep-burial settings because of elevated temperatures, fluid mixing, and chemical compaction (see above). Cementation of several volume percent, which is common, requires large fluid fluxes, however, because the solubility of most carbonates is rather low (e.g., K = 10 -8.52 at 25°C for calcite, decreasing with increasing temperature, Robie et al., 1979). Nevertheless, numerous case studies have demonstrated extensive intermediate- and deep-burial cementation (and concurrent porosity/permeability losses) in carbonate aquifers, not only with carbonate minerals but also with sulfates, sulfides, and clay minerals (e.g., Scholle and Halley, 1985; Walls and Burrowes, 1985; Woronik and Land, 1985; several articles in Montañez et al., 1997). By contrast, some studies have shown deep burial cementation to be relatively minor (e.g., Prezbindowski, 1985).
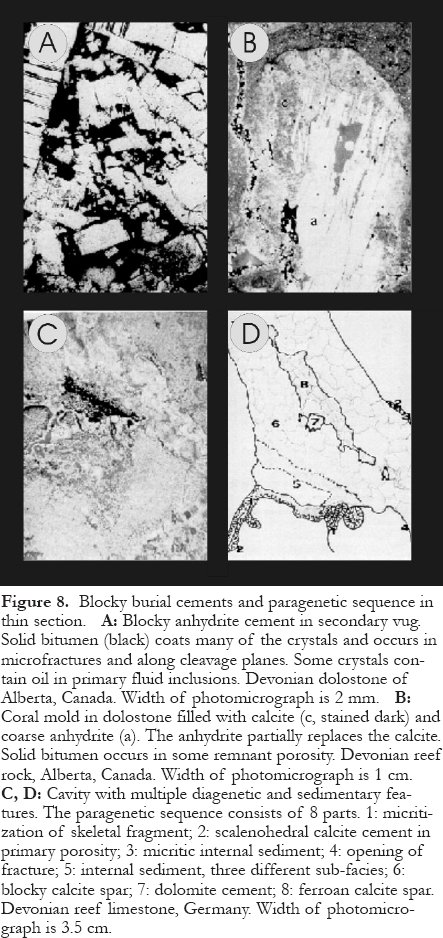
40 Burial cements in carbonate rocks are mainly calcite, dolomite, and/or anhydrite, and they have a number of characteristics: (a) crystals cross-cut stylolites, or microstylolites and pressure solution seams terminate at cement crystals; (b) crystals occur in fractures or spalled ooid cortices; (c) crystals fill compacted pores; (d) crystals enclose compacted grains; (e) crystals contain hydrocarbons in fluid inclusions; (f) crystals partially replace earlier burial cements; (g) crystals have relatively large size (diameters from several tens of micrometres up to several centimetres); (h) crystals are ‘ blocky' and form equant mosaics, increase in mean diameter toward pore centres, or are poikilotopic (poikilitic); (i) crystals are not recrystallized; (j) carbonate crystals show well-defined cathodoluminescence zonation that ranges from bright towards quenched (dull), in some cases repeatedly, or they are dull-luminescent throughout, commonly caused by ferroan composition (divalent iron >500 ppm); (k) carbonate crystals have oxygen isotope ratios that are depleted relative to marine cements of the same stratigraphic age as the host rocks; and (l) crystals contain two-phase fluid inclusions with elevated temperatures of homogenization (>50°C) and large freezing point depressions (indicating high salinities). Characteristics (a) to (f) above are distinctive and indicate a burial origin (Figs. 7 and 8), but are relatively rare in some rocks. The other characteristics are not unique to burial diagenetic cements. Therefore, any interpretation of burial cementation should combine as many lines of evidence as possible.
Figure 5. Schematic representation of pressure solution contacts between grains of different ‘ pressure solution solubility'. Note that limestone grains are penetrated by dolomite and chert, dolomite is penetrated only by chert. Contacts between grains of equal ‘ solubility' tend to be serrated (stylolitic), contacts between grains of unequal ‘ solubility' tend to be smooth. Modified from Trurnit and Amstutz (1979).Figure 6. Schematic representation of pressure solution contacts depending on the induced stress at the crystal-crystal interface for several mineral pairs. The contours denote interfacial normal stress generated by growth driven by a supersaturation of OMEGA=2 for phase A (the first listed in any given pair) at T=100 °C. For details of the calculation procedure see Fletcher and Merino (2001). This figure is modified from their paper.Dissolution
41 Dissolution is common in intermediate-and deep-burial settings (albeit volumetrically it is not as important as cementation, or else a general porosity decrease with depth would not occur - see Figure 3), especially near the oil window where decarboxylation and certain other mineral reactions generate acidity. Secondary porosity is well known from carbonates (Choquette and Pray, 1970) and clastics (Schmidt and MacDonald, 1979). Significant dissolution may or may not involve large fluid fluxes. If acidity is generated within or near the rock with secondary porosity, large-scale pore water flow is not necessary.
42 A hitherto controversial process to generate secondary porosity in deep burial settings is thermochemical sulfate reduction (TSR). Model calculations suggest that partial dissolution of carbonate rocks may occur at least initially during TSR (Hutcheon, 1992; Nicholson, 1992), but most published case studies of TSR do not show a significant increase in porosity (e.g., Machel et al., 1995a). Dissolution via TSR, however minor, usually is an in situ process because TSR commonly takes place in hydrologically closed or nearly closed systems (e.g., Machel 2001).
Recrystallization
43 The matrix, allochems, and cements formed at shallow depths become thermodynamically metastable or unstable with increasing burial because of increasing temperature, pressure, and changing composition of the groundwaters. Barring kinetic inhibition, recrystallization and replacement of metastable and unstable minerals will occur. However, porosity and permeability are generally little affected by recrystallization.
Figure 7. Bladed to fibrous burial cements. A: Multiple generations of radiaxial fibrous calcite cement filling subvertical fracture. Compactional or tectonic stresses resulted in multiple micro-faulting roughly perpendicular to the orientation of the fracture. Devonian reef limestone, Germany. Width of photograph is 3 cm. B: Multiple generations of radiaxial fibrous calcite cement and internal sediment filling subvertical fracture. In this case, the fracture was completely filled by the cement but was re-opened, as indicated by the fragment of fibrous calcite (solid arrow) that was torn off (hollow arrow) and re-deposited within the internal sediment. Devonian reef limestone, Germany. Width of photograph is 6 cm. C: Subhorizontal fracture in fine-grained dolostone (black, top and bottom). Fibrous anhydrite with distinctive inclusion trails fills the fracture, indicating that the fracture was filled concomitantly with subvertical dilation by the crack-seal mechanism (Machel, 1985). Devonian dolostone, Alberta, Canada. Width of photograph is 7.5 cm.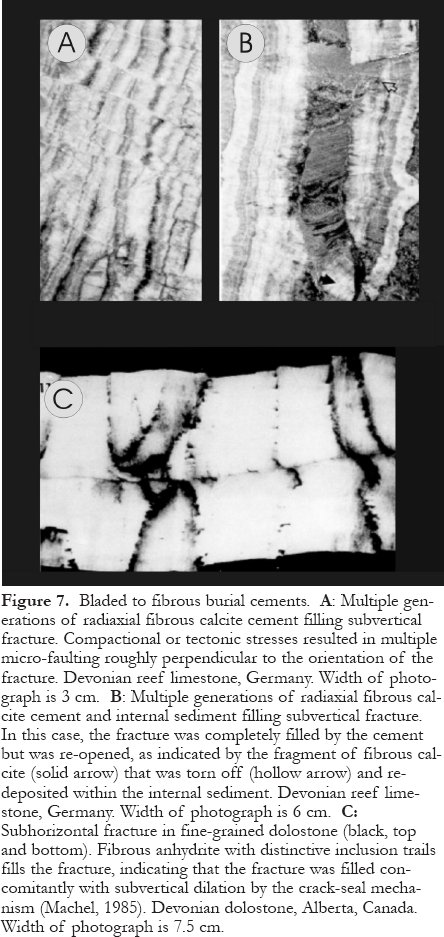
Display large image of Figure 7
Display large image of Figure 8
44 The most common textural manifestations of recrystallization of carbonate cements are the enlargement of crystals (Ostwald ripening) and partial elimination of primary crystal or grain boundaries (Figs. 9A, B). Cathodoluminescence images of recrystallized carbonates commonly are ‘ blotchy'. Recrystallization also results in changes in crystal structure, isotopic composition, and paleomagnetic properties (e.g., Machel, 1997).
45 For practical applications, the absence, presence and/or degree of recrystallization is/are important for genetic interpretation. Consider, for example, a dolostone. If recrystallization changes or resets the original properties of the cystals, such as texture, structure, composition, and/or paleomagnetic properties, beyond what they were in the pristine (unrecrystallized) rock, the dolostone is said to be "significantly recrystallized". The reset properties are no longer representative of the fluid and environment during dolomitization but they reflect the recrystallization event. However, some measurable properties may not be reset and they will represent the dolomitization event. If recrystallization resets only one of 10 properties (e.g., 87 Sr/86 Sr-ratios) beyond the range in the pristine rock, the dolostone is significantly recrystallized with respect to Sr-isotopes, yet insignificantly recrystallized with respect to the other nine properties. In either case, values of those properties that are identical to the pristine reference values can be used for genetic interpretations of dolomitization.
46 The concept of "significant recrystallization" is of great use in genetic interpretations. In particular, for a reliable interpretation of the chemistry of the diagenetic fluids, it is sufficient to recognize that a crystal is insignificantly recrystallized (Machel, 1997).
Replacement
47 Next to dolomite (discussed separately below), the most common replacive mineral in deep carbonate aquifers, is anhydrite. For example, extensive anhdrite replacement of dolostones, with or without concomitant anhydrite cementation, is well known from the Devonian strata of western Canada (e.g., Walls and Burrowes, 1985). Replacive anhydrite commonly is massive but may also resemble primary nodular or bedded sulfates, which is best recognized where reef rock is replaced (Fig. 9C). At depths of less than 600m (up to 1000m depending on temperature and fluid composition), gypsum forms rather than anhydrite because the gypsum-anhydrite stability boundary is crossed somewhere in this interval. Furthermore, replacive gypsum generally dewaters and recrystallizes to anhydrite, generating distinctive microtextures (Fig. 9D).
Figure 9. Common recrystallization (A, B) and replacement (C, D) textures. A, B: Recrystallized coral (c) and fibrous calcite cement (fi) (A: single-polar, B: crossed-polar photomicrograph). Note than all crystal boundaries appear ‘ diffuse' and that large crystals consist of numerous subcrystals. The dark domains at top left and right are intraparticle and interparticle primary pores, respectively, filled with micritic sediment. Devonian reef limestone, Germany; width of photomicrographs is 0.5 cm. C: Massive, white anhydrite replacing coral dolostone, a part of which is preserved at centre-right. Devonian reef dolostone, Alberta, Canada; width of photograph is 6 cm. D: Thin section photomicrograph, crossed polars, of anhydrite shown in Figure C. Large, marginally corroded porphyrotopic crystals are embedded in fine-crystalline matrix [‘ corrotopic anhydrite']. This texture is typical of gypsum recrystallized to (replaced by) anhydrite.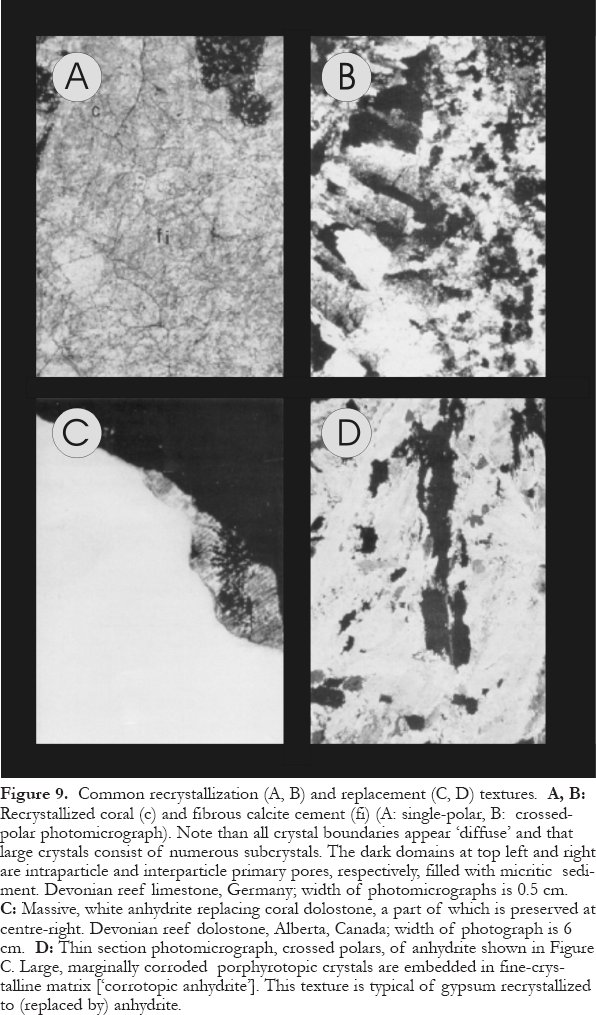
Display large image of Figure 9
48 Dolomite and silica (chert) are other common replacements of limestone. The resulting textures can be predicted from thermodynamic considerations to some degree. As Fletcher and Merino (2001) have shown, the induced stress by quartz growing replacively toward and within calcite is very small (Fig. 6); hence, quartz tends to form euhedral crystals in limestone. The same is true for dolomite in limestone, at least at relatively low temperatures. However, where gypsum grows in limestone, the induced stress at the grain boundaries is very large; thus, calcite tends to deform or fracture, unless there is enough porosity to accommodate the sulfate.
49 Replacement of carbonates by calcium sulfate can have a pronounced effect on porosity and permeability. Regardless of the actual quantities of anhydrite formed relative to dolomite replaced or cemented, dolostones commonly are porous, whereas anhydrite is tight. Hence, any anhydrite cement or replacement will reduce the porosity significantly. Permeability, however, is not necessarily affected to a proportional degree. Anhydrite generally has a low nucleation rate and thus a tendency to grow in a few patches of large crystal aggregates, rather than forming pervasively throughout the rock. As a result, more than about 60% of the total rock volume has to be filled or replaced by anhydrite cement before the permeability is reduced to any significant degree (Lucia et al., 2004). At lesser amounts of anhydrite in the rock, the formation fluids (water and/or petroleum) tend to flow around the large patches of anhydrite.
Epimetamorphic Fluids and Tectonic Injection
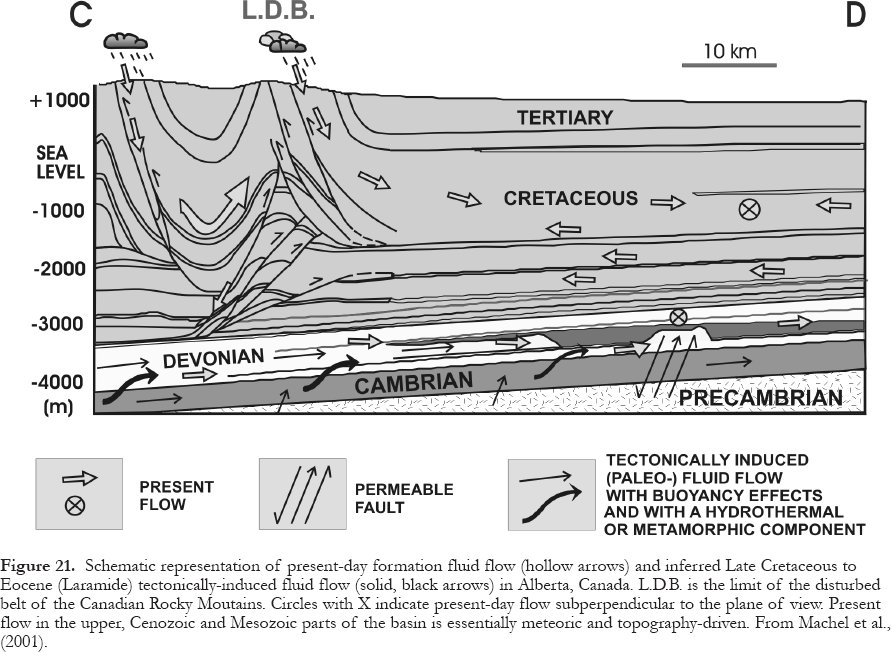
50 Diagenesis in the deepest parts of the diagenetic realm is a poorly understood phenomenon because it grades into the epimetamorphic realm. Some authors (Land, 1997; Schroyen and Muchez, 1998) advocate that material can be transferred from the crystalline basement into the overlying deep burial diagenetic setting. Prograde metamorphism and devolatilization reactions liberate water and CO2 , which are added to the sedimentary basin above. In the case of the Western Canada Sedimentary Basin, there is petrographic, isotopic, and circumstantial evidence for injection of Laramide metamorphic fluids into the Devonian carbonate aquifers in the deepest part of the basin (Machel and Cavell, 1999). The fluid fluxes appear to be low, however, as shown by the relatively small amounts of cement formed, commonly white sparry calcite (Machel et al., 2001). The effects of this type of fluid flow on reservoir properties are not yet understood.
Processes and Features Occurring and/or Forming Across Several Burial Diagenetic Zones
Fractures
51 Where fractures are restricted in lateral or horizontal extent within one of the diagenetic burial zones (Fig. 2), diagenesis within the fractures is generally similar or identical to that within the adjacent wall rocks. Where fractures cross from one diagenetic zone into another, however, relatively cold groundwaters may descend or relatively hot groundwaters may ascend fairly rapidly. Hence, the fractures in any diagenetic zone may contain hydrofrigid (and underpressured) or hydrothermal (and overpressured) fluids relative to those in the surrounding wall rocks (Machel, 1999; Machel and Lonnee, 2001). This situation raises numerous possibilities for mineral reactions, especially when the fractures transect different rock types (e.g., Pedersen and Bjørlykke, 1994; Stoute and Harris, 1995). A comprehensive discussion of these possibilities is beyond the scope of this paper. The following examples characterize the most common and perhaps most important aspects of formation water flow though fractures.
52 Rapid descent of cool meteoric waters to depths of several kilometres through deep-reaching faults/fractures is common in mountainous recharge areas. In Western Canada, for example, such hydrologic systems are common in the Rocky Mountain Front Ranges, where meteoric waters convectively descend and return to the surface in sulfurous hydrothermal springs, e.g., at Miette, Banff, and Radium. The diagenetic effects in the descending parts of these flow systems are virtually unknown, except that the waters appear to equilibrate isotopically rather fast in at least some locations (Nesbitt and Muehlenbachs, 1997). The major effect in the ascending parts of these flow systems is massive cementation within the fractures, which commonly reach centimetre and locally decimetre dimensions, and are filled with sparry calcite or dolomite, quartz, and to a minor degree with other minerals, including sulfates and fluorite (Nesbitt and Muehlenbachs, 1997).
53 Similarly, from a hydrogeological point of view, rapid convection of meteoric waters is known from intermontane rift basins. In one of the best studied examples, Tóth and Otto (1994) demonstrated that meteoric waters move preferentially through fractures from recharge areas in the mountains that fringe the Rhine Graben toward the enclosed valley, where they discharge as hydrothermal and petroliferous brines. Radiocarbon data from waters of producing wells range from 3,165 to 31,000 years, indicating that convection is very rapid. The diagenesis within the fractures has not been investigated, however.
54 Rapid ascent of hydrothermal fluids derived from shallow-metamorphic and deep-burial diagenetic settings is well known from many sedimentary basins. Perhaps the best known effect of such "hydrothermal groundwater flow" is Mississippi-Valley-Type Pb-Zn-(saddle) dolomite mineralization that may be restricted to fractures, or that may partially replace adjacent wall rocks (e.g., Anderson and Macqueen, 1982; Braithwaite and Rizzi, 1997; Gregg et al., 2001).
55 Fibrous minerals in fractures indicate a special mode of fracture opening and filling, commonly referred to as "crack-seal" (Ramsey, 1980). In this case, the pore fluids precipitate minerals as fast as the fractures are opening, which may happen in multiple episodes, such that the actual space between the facture walls never exceeded a few nanometres or micrometres (Machel, 1985). An example of fibrous anhydrite is shown in Figure 7C. Ramsey (1980), Machel (1985) and others have interpreted the fibrous crystals to be passively precipitated in veins that opened under externally applied stress. An alternative interpretation has been provided by Wiltschko and Morse (2001), who advocated that such fibrous mineral veins originate from the vein mineral(s) actively pushing the wall rock apart.
56 Fractures commonly serve as migration pathways for petroliferous fluids. In such cases, the fluids may form minerals with petroliferous fluid inclusions and/or solid bitumen in the fractures, which can be used to determine hydrocarbon migration pathways and related aspects, such as timing of migration and fluid composition (e.g., Levine et al., 1991). In deeply buried and tightly sealed reservoirs, fractures are not only used by hydrocarbons as migration pathways but they may be generated by the pressure from in-situ hydrocarbon generation, either oil or gas from kerogen, or gas from oil. Especially well documented examples of this phenomenon occur in Devonian reefs in the deepest part of the Alberta Basin, Canada, where in-situ cracking of oil to gas has led to intense hairline fracturing that appears as pervasive "shattering" of the rocks (Marquez and Mountjoy, 1996; Fig.10).
Bacterial and Thermochemical Sulfate Reduction
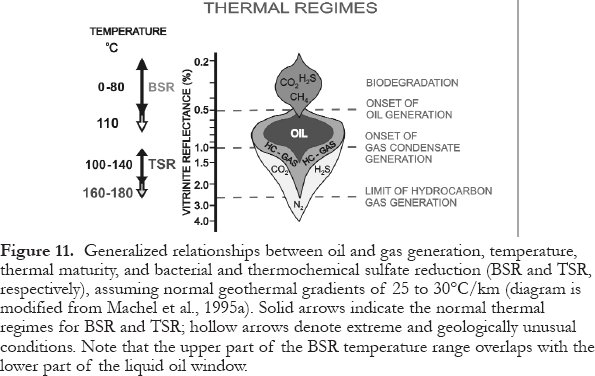
57 Dissolved sulfate and hydrocarbons are thermodynamically unstable together in virtually all diagenetic environments. Hence, redox-reactions occur, whereby sulfate is reduced by hydrocarbons either bacterially (bacterial sulfate reduction = BSR) or inorganically (thermochemical sulfate reduction = TSR). Their geological and economically significant products are similar and can be formed in or across several burial zones. Based on empirical evidence, BSR and TSR occur in two mutually exclusive thermal regimes (Fig. 11): low-temperature and high-temperature diagenetic environments, respectively (Machel et al., 1995a; Machel, 2001).
58 BSR is common in diagenetic settings from zero to about 80°C. Above this temperature range, almost all sulfate-reducing microbes cease to metabolize, although they may persist in a dormant state. Those few types of hyper-thermophilic microbes that can form H2S at temperatures up to 110°C appear to be very rare and they do not normally occur and/or metabolize in geologic settings that are otherwise conducive to BSR.
59 TSR appears to be common in geologic settings with minimum temperatures in the range of 100 to 140°C, while in some settings minimum temperatures of up to 180°C are necessary. TSR does not have a sharply defined minimum temperature because the onset and rate of TSR are governed by several factors that vary from place to place. These factors include the composition of the available organic reactants, kinetic inhibitors and/or catalysts, anhydrite dissolution rates, wettability, as well as migration and diffusion rates of the major reactants toward one another (Machel, 1998).
Figure 10. Microfractures in polished core samples from deeply buried and tightly sealed carbonate reservoirs in Alberta, Canada. A: Dense network of microfractures filled with bitumen (black). cm-ruler for scale. B: Subhorizontal microfractures, bitumen filled (arrow), in coral fragment. The fractures stop at the boundary with the adjacent bladed cement (c), presumably because the latter was more ductile. Scale bar = 250 micrometres. C, D: Subvertical and subhorizontal sets of bitumen-filled microfractures. Scale bar = 250 micrometres. E : Microfractures distributed radially around nearly spherical mold (arrow) in dolomitized reef rock. In addition, multiple sets of subhorizontal microfractures extend from intercrystalline pores and dissolution vugs into the matrix and adjacent fossil fragments.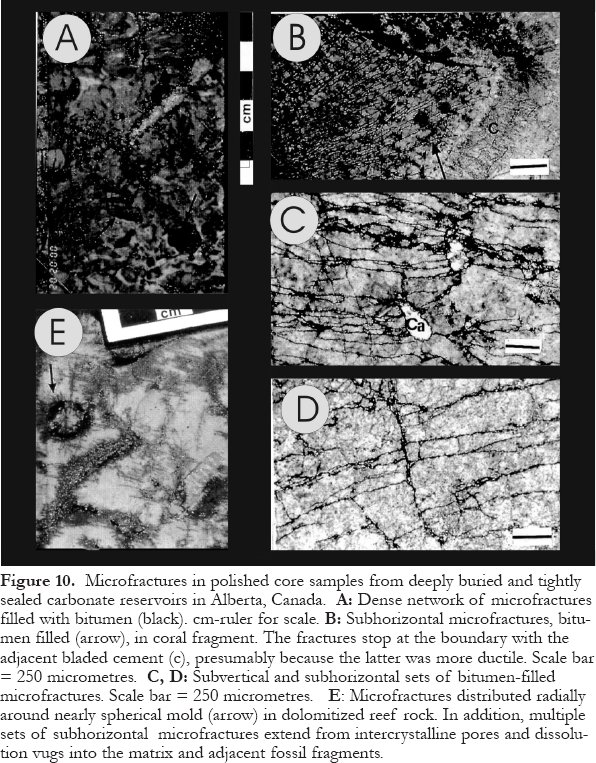
Display large image of Figure 10
Display large image of Figure 11
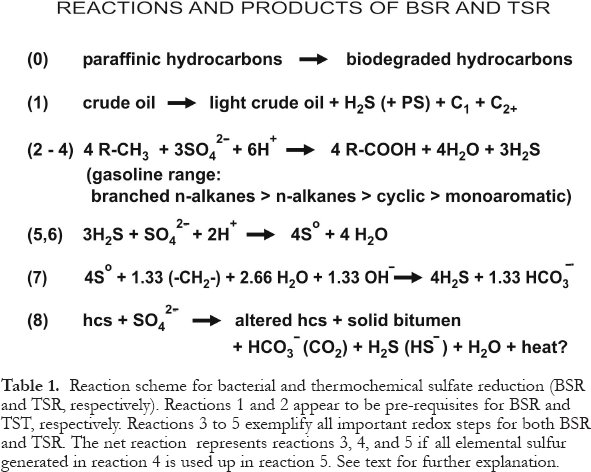
60 The most important redox-reactions involving BSR and TSR can be represented by a common set of mass and charge balance reactions (Table 1). All reactions representing inorganic processes are reversible but are shown only in that direction to which they proceed under the specified conditions. Each microbial reaction involves numerous enzyme-catalyzed steps that are omitted for clarity. Reactions 1 and 2 are specific to BSR and TSR, respectively, whereas most redox-reactions involving hydrocarbons and inorganic sulfur compounds are valid for both BSR and TSR. For a more comprehensive discussion of the geological, experimental and theoretical evidence for these reactions, see Machel (1987) and Machel et al. (1995a).
61 The main organic reactants for BSR are organic acids, methane, and other products of aerobic or fermentative biodegradation. The main organic reactants for TSR are branched and nalkanes, followed by cyclic and monoaromatic species, in the gasoline range. Sulfate is derived almost invariably from the dissolution of primary or secondary gypsum and/or anhydrite at or near the redox-reaction site(s).
62 The products of BSR and TSR are similar, but their relative amounts vary widely and are determined by a number of locally variable factors, including availability of reactants, formation water chemistry, and wettability (Machel, 1997, 2001; Machel et al., 1995a). The primary inorganic reaction products in both thermal regimes are H2S (HS - ) and HCO3 - (CO2 ). Carbonates, particularly calcite and dolomite, commonly form if alkali-earth metals are present in the formation waters. Other carbonates, i.e., ankerite, siderite, witherite, strontianite, may form if the respective metal cations are available. Iron sulfides, galena, and sphalerite form as by-products of hydrogen sulfide generation, if the respective transition or base metals are present or transported to the BSR/TSR reaction site. Elemental sulfur may accumulate as a volumetrically significant net reaction product, generally when the system runs out of reactive hydrocarbons. Water may form as a by-product and can cause a local dilution of the formation waters at or near the reaction site. There are case studies of TSR, however, where no dilution of the formation water has occurred, indicating that the amount of water released during TSR was negligible. Porosity may be generated during TSR, but most case studies show that TSR does not normally increase porosity significantly.
63 TSR is likely to take place in fairly narrow reaction zones, where the irreducible water saturation in the hydrocarbon-containing pores is low. In the Nisku Formation of Alberta, Canada, this zone is about 10 - 20 m thick (Machel et al., 1995b). However, where the irreducible water saturation is high, TSR may take place throughout the entire hydrocarbon-containing pore volume. Solid bitumen may form as a byproduct of both BSR and TSR. Finally, the net reaction may be exothermic or (more commonly) endothermic, depending on reaction stoichiometry (Simpson et al., 1996; Simpson, 1999).
64 The mere presence of any of the above reaction products and by-products does not permit a distinction between BSR and TSR. However, a number of petrographic relationships and geochemical criteria can be used to discriminate between these two processes (Machel et al., 1995a). Specifically, most solid products of BSR and TSR, although similar in gross composition, can be distinguished from one another petrographically. Most BSR products form early, whereas most TSR products form late, in the paragenesis, and have distinctive crystal sizes, shapes, and/or reflectivity. However, there also are cases where BSR products formed relatively late diagenetically, such as in uplifted reservoirs after hydrocarbon migration. Gas chromatography, δ13 C-, δ34 S-analyses, and/or a combination thereof, offer the best distinguishing geochemical criteria (Machel et al., 1995a).
Table 1. Reaction scheme for bacterial and thermochemical sulfate reduction (BSR and TSR, respectively). Reactions 1 and 2 appear to be pre-requisites for BSR and TST, respectively. Reactions 3 to 5 exemplify all important redox steps for both BSR and TSR. The net reaction represents reactions 3, 4, and 5 if all elemental sulfur generated in reaction 4 is used up in reaction 5. See text for further explanation.
Dolomitization
65 One of the most important effects of groundwater flow on carbonate aquifers and potential reservoir rocks is pervasive replacive dolomitization, which commonly but not necessarily results in a concomitant increase in porosity and permeability. Mass balance calculations indicate that large fluid fluxes are necessary for extensive, pervasive dolomitization, because of the generally low Mg-content of natural waters. From 6,500 to 10,000 m3 of brackish to fresh groundwaters (the former containing about 10% seawater) would be required to dolomitize one cubic metre of limestone with an initial porosity of 40%, or 650 m3 of seawater, the most common Mg-rich water in nature, would be required (Land, 1985). Porosity generally increases during dolomitization because generally about two moles of calcite are replaced by one mole of dolomite, with a concomitant decrease in the molar volume by about 13%. Other reaction stoichiometries, however, may result in little porosity change or in porosity loss during dolomitization (e.g., Machel and Mountjoy, 1986). Also, continued flux of fluids supersaturated with respect to dolomite may lead to cementation of pore spaces with dolomite after complete matrix replacement, a process termed "overdolomitization "(Lucia 2000). In extreme cases, the resulting dolostones are essentially non-porous and impermeable.
66 Massive dolostones with enhanced porosity and permeability relative to their limestone precursors are common all over the world. Popular dolomitization models (Fig. 12) typically depict limestones embedded in some type of local, intermediate, or regional groundwater flow system that is invoked to pump the necessary amounts of Mg through the rocks (various articles in Purser et al., 1994; Mountjoy et al., 1997; Machel, 2004). Thereby, the distribution, texture, and geochemical composition of the dolostones are fitted to perceived or possible models, in an attempt to obtain a viable genetic interpretation. Such models span all diagenetic settings from near-surface to the metamorphic realm, as well as almost any type of hydrologic flow system and geochemical composition.
Microbial Action
67 Most microbes live in near-surface or shallow subsurface diagenetic settings where temperatures and pressures are relatively low and nutrients are abundant. Temperature and pressure increase with increasing depth and nutrients become scarce, which leads to a general decrease in the rates of metabolic activity and eventually to death of all microbes. The various types of microbial activity and products in petroliferous subsurface settings have recently been summarized by Machel and Foght (2000), in an attempt to place absolute depth limits on the geologically significant groups of microbes.
Figure 12. Diagrams of several groundwater flow systems (left) and predicted dolomitization patterns (right). Examples are of incomplete dolomitization of carbonate platforms or reefs. Arrows denote flow directions; dashed lines show isotherms. Predicted dolomitization patterns are shaded. Models A-D1 and D4 are km-scale; model D2 and D3 are basin-scale. Reproduced from Machel (2004).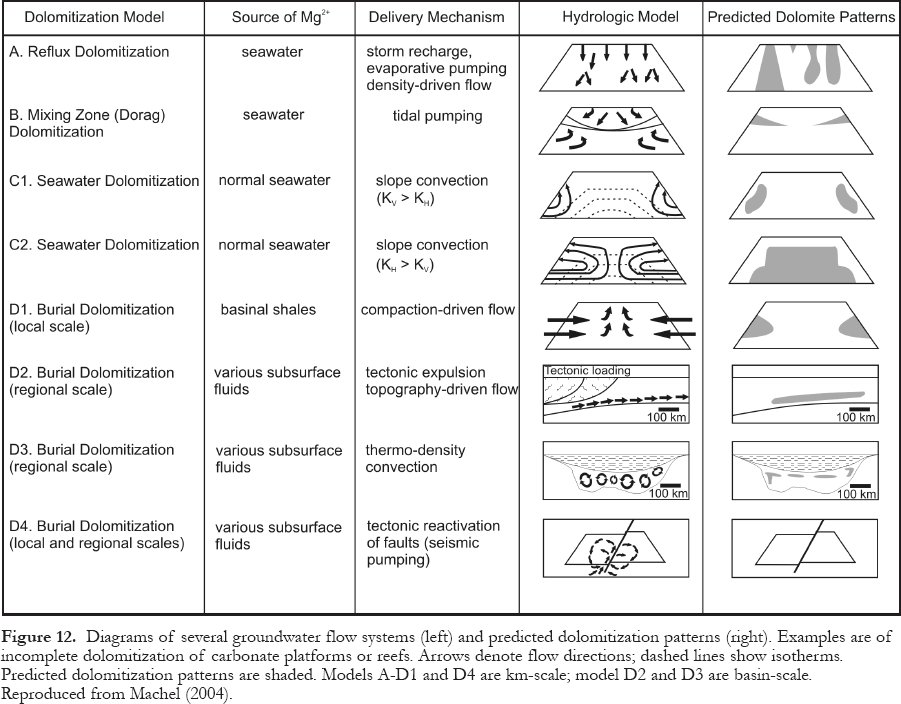
Display large image of Figure 12
68 Four groups of microbes are known to be geologically significant in petroliferous subsurface settings (Machel and Foght, 2000), namely aerobic respiratory bacteria, and three anaerobic groups that commonly live in consortia (symbiotic communities): fermentative, sulfate-reducing, and methanogenic bacteria. These microbes form a number of economically important products and by-products in subsurface settings. The aerobic microbes, generally called biodegrading bacteria, form mainly napthenic crude oils and tar in the form of tar sand deposits (Tables 2 and 3). The role of fermentative microbes is mainly in the partial breakdown of organic molecules that then serve as nutrients for the sulfate reducers and the methanogens. Sulfate-reducing microbes form mainly H2 S, metal sulfides, and elemental sulfur, wheras methanogenic bacteria form mainly methane. In all cases, carbonate cements with distinctive isotopic compositions may be formed as by-products. In addition, nanobacteria may be important in clay mineral diagenesis in buried sandstones. Various types of microbes from the above groups can be used for microbially enhanced recovery of oil. Ultramicrobacteria constitute a special class, as they are injected in a dormant state and then resuscitated in situ to form biobarriers.
69 In the subsurface, temperature appears to be the principal factor limiting microbial metabolism (and life), followed by the availability of suitable nutrients. All four groups of bacteria generally become inactive by a subsurface depth of about 2000 m, except for rare cases where aerobic biodegradation or sulfate reduction can occur as deep as about 3000 m (Machel and Foght, 2000). Sulfate-reducing bacteria appear to be able to tolerate the highest temperatures, up to at least 110°C. However, they normally are subject to the same depth limitations as the aerobic biodegraders (Fig.13), whose "waste" products they may use as nutrients. Similarly, most methanogenic bacteria appear to become dormant or are dead at depths greater than about 2000 m (Fig. 14). This, however, does not preclude that sulfate-reducing and/or methanogenic microbes may metabolize in rare and unusual cases at greater depths. Furthermore, the lower limit of the biosphere probably is marked by other types of bacteria or archeae at much greater depths.
Porosity and Permeability in Limestone-Dolostone Sequences
70 Porosity and permeability development transgresses all diagenetic zones and is a direct result rather than a process of diagenesis. For petroleum engineering, this development is the most important aspect, especially when comparing limestone with dolostone sequences.
71 It has long been claimed that most dolostones are more porous and more permeable than limestones (Van Tuyl, 1914; Blatt et al., 1972), a circumstance of obvious importance for the petroleum industry. A related aspect is that dolostones generally form aquifers and preferential migration pathways for hydrocarbons, or both. However, Schmoker and Halley (1982) and Halley and Schmoker (1983) demonstrated that many dolostones have porosities equal to or less than those of adjacent limestones, based upon porosity/depth profiles of Cenozoic carbonates in southern Florida, which have not been buried too deeply (less than 1 km). Budd (2001) showed that the same is true for the permeability in these particular rocks, i.e., many dolostones have permeabilities equal to or less than those of the adjacent limestones. On a larger scale, Schmoker et al. (1985) compared thousands of limestones and dolostones from across the U.S.A. and found that dolostone reservoirs commonly have lower matrix porosities and permeabilities, yet higher fracture porosities and permeabilities, than limestones. Amthor et al. (1994), however, in a study of 31 wells from Devonian reservoirs in Alberta that span a depth range of several thousand metres, found that there is a distinctive dependence on depth. If considered irrespective of depth, limestones and dolomitic limestones are more porous than dolostones, whereas at burial depths of greater than 2000 m dolostones are significantly more porous and permeable than limestones. There also are notable examples of very young, near-surface dolostones that are tight and apparently devoid of porosity, as in the Plio-Pleistocene carbonates of Bonaire (Lucia and Major, 1994).
Table 2. Aerobic biodegradation effects on gross properties of petroleum (modified from Connan, 1984).

Display large image of Table 2
Table 3. Changes in molecular properties with increasing level of aerobic biodegradation (compiled from Volkman et al., 1983; Connan, 1984; Hunt, 1996).
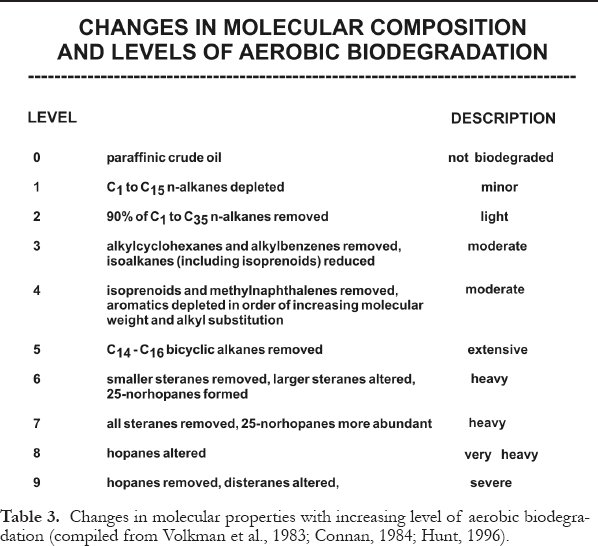
Display large image of Table 3
72 The major processes involved in porosity-permeability (poroperm) generation are summarized in Figure 3. Six specific processes are especially important in the case of limestone-dolostone sequences: (a) mole-per-mole replacement; (b) dissolution of unreplaced calcite (solution undersaturated for calcite after all Mg in excess of dolomite saturation is exhausted); (c) dissolution of dolomite (without externally controlled acidification); (d) acidification of the pore waters (via decarboxylation, clay mineral diagenesis, etc.); (e) fluid mixing (Mischungskorrosion); and, (f) thermochemical sulfate reduction, which may generate porosity under certain circumstances (Machel, 2001, 2004). The wide scatter and lack of systematic relationships between the porosity of limestones and dolostones in Florida and Alberta probably reflects locally and regionally heterogeneous interplays among the various processes that generate, preserve, or destroy porosity (Fig. 3).
73 Dolomitization almost invariably involves the reorganization of permeability pathways. Commonly permeability increases along with porosity, and vice versa. This is documented through studies such as the one on the Late Devonian Grosmont Formation in eastern Alberta, which hosts a giant heavy-oil reservoir (Luo et al., 1994; Luo and Machel, 1995; Machel and Huebscher, 2000). A comparison of porosity and permeability data from 237 core plugs reveals an overall positive correlation, despite considerable scatter. This correlation is also expressed in the displacement pressures from mercury injection capillary measurements, which permit the identification of four major and two minor dolomite reservoir rock types. Using a similar approach (thin section and SEM petrography combined with helium porosimetry and mercury injection capillary measurements), Woody et al. (1996) also documented positive and statistically significant correlations between porosity and permeability for planar dolomites in the Cambrian-Ordovician Bonneterre Formation of Missouri, U.S.A., which is host to one of the world's largest MVT-sulfide deposits.
Figure 13. The difference in sulfur isotope composition between sulfate dissolved in formation waters and hydrogen sulfide versus depth from three sedimentary basins (after Krouse, 1977). High isotope values at depths less than about 2000 m indicate in situ BSR, the effect of which diminishes with increasing depth. At depths below about 2200 m, BSR appears to be volumetrically insignificant and all H2 S is thermochemical.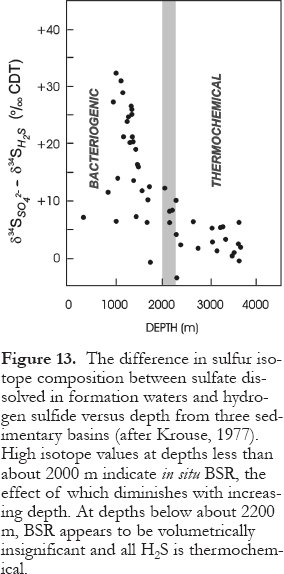
Display large image of Figure 13
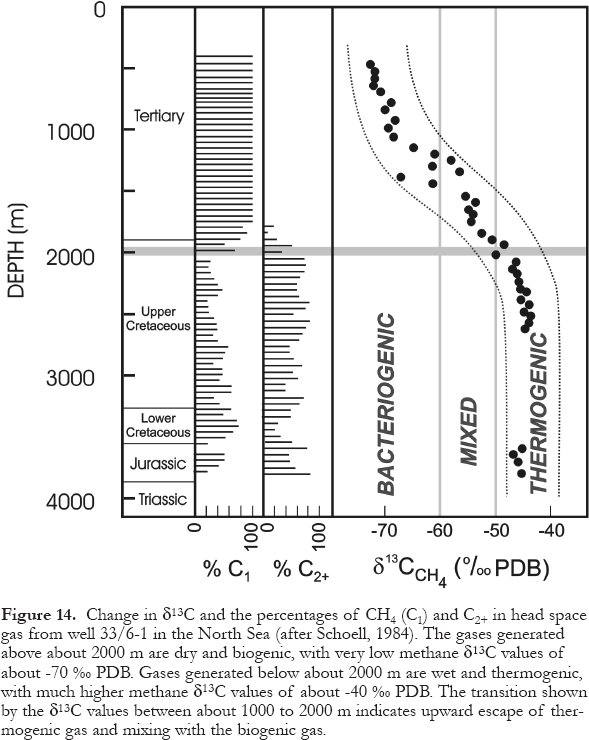
Display large image of Figure 14
74 Other authors have disputed that there is a systematic correlation between porosity and permeability in dolostones, or that these two petrophysical parameters are enhanced in dolostones relative to limestones. Halley and Schmoker (1983), in the absence of reliable or sufficient permeability data, attempted to assess the permeability of carbonate rocks from porosity data. They found that carbonate aquifers and carbonate aquicludes cannot be distinguished on the basis of porosity. Lucia (2002, 2004) claimed that "....there is no relationship between porosity and permeability in dolostones...... and dolomite crystal sizeand the precursor fabric are key elements in predicting permeability", and "Dolomitization of grain-dominated limestones usually does not change porosity-permeability relationships. Instead, the precursor fabric controls pore-size distribution." While this may be so in some, perhaps many cases, the Grosmont and the Bonneterre examples clearly show that there is a relationship between porosity and permeability in at least some major and economically important dolostone sequences.
THE "6 - STEP PROCESS"
75 This writer has found that many investigations of burial diagenesis, whether of carbonates or of clastics, proceed in six steps that roughly correspond to those in the top half of Figure 15. However, such studies should proceed in the "6 -Step Process" shown in the bottom half of Figure 15, with the last step being optional.
76 Step 1: Facies analysis (outcrop, core, thin section). Facies analysis is necessary because depositional factors and processes, such as bathymetry and water agitation, determine the primary poroperm distribution. These, in turn, control the extent to which diagenetic fluids later permeate the rocks, creating secondary porosity, cements, replacements, etc. An example of a facies analysis is shown in Figure 16. Facies analysis should be preceded by, or done in combination with, characterization of stratigraphy and structure for an overall 3D context, if possible.
77 A commonly neglected aspect is the "primary aquastratigraphy", which is herein defined as the syn-sedimentary pore fluid stratification that can be correlated with, or deduced from, the facies analysis. The primary aquastratigraphy is extremely important for diagenetic investigations. For example, the sedimentary sequence depicted in Figure 17, would have contained, at the time of deposition, normal seawater in the lower part, meteoric water in the middle and evaporitic brine at the top. The low-density meteoric water would not be able to displace the underlying seawater, unless an external hydrologic force would overcome the density difference. However, the evaporitic brine would move downward and displace both the meteoric water and the seawater in the sedimentary sequence, probably leading to an evaporitic diagenetic overprint very soon after deposition, including changes in the isotopic and trace element compositions. Such phenomena would remain undetected and/or misinterpreted without a proper facies analysis.
78 Step 2: Establish the paragenetic sequence of diagenetic events (Fig. 18) on the basis of petrography (outcrop, core, thin section, including UV and CL microscopy). This may involve the identification of shallow and deep burial cements using petrographic criteria, cement sequence (cement stratigraphy), intervening episodes of deposition of internal sediment(s), fracturing, dissolution, and recrystallization. Under favorable circumstances, broad depth limits can be assigned, such as less than 10 m for sea floor diagenetic processes, less than 300 m for processes predating stylolitization, etc. (as discussed in Machel, 1999). Investigation of cements and porosity in core and thin section can be combined with extant poroperm data from plugs and full diameter core, in order to map out porosity and permeability on a reservoir scale.
79 Step 3: Obtain various physical (P/T, crystal structure) and geochemical (isotopes, trace elements) and/or paleomagnetic data for selected diagenetic phases of interest, such as replacive dolomite(s), sparry calcite cements, etc. Most rock geochemical methods involve microdrilling (in the old days wholesale milling) to obtain powder samples for subsequent geochemical analysis. Such geochemical work is commonly referred to as "bulk analysis". Every attempt should be made to extract individual diagenetic phases, which is possible by these methods only with relatively large crystals. To achieve higher resolution and/or analyze individual growth zones, spot analyses by microbeam techniques may be carried out. With modern technology, spot sizes are commonly smaller (a few tens of micrometres) than the width of typical cement growth zones. However, this type of work requires expensive and generally unobtainable instrumentation. A further problem is that some of the desired analyses are notoriously difficult or impossible to obtain with sufficient accuracy using microbeam techniques, such as the light isotope ratios (oxygen, carbon), and detection limits for trace elements usually are much higher than by bulk analytical techniques. Hence, a compromise has to be struck. Some types of analysis are entirely non-destructive, such as cathodoluminescence imagery and spectroscopy, or microthermometry of fluid inclusions. The available data are then depicted in a combination of diagrams (an example is shown in Fig. 19), and are interpreted in terms of fluid composition, temperature, etc. Where formation fluids can be obtained from well heads, an attempt can be made to relate the present formation water chemistry to that inferred from the rock geochemistry. Data from Step 3 are then integrated into the paragenetic sequence (Fig.18) and, where possible, absolute timing is assessed using radiometric or paleomagnetic data.
Figure 15. The "6-Step Process", normal and recommended phases of a project.
Display large image of Figure 15
Display large image of Figure 16
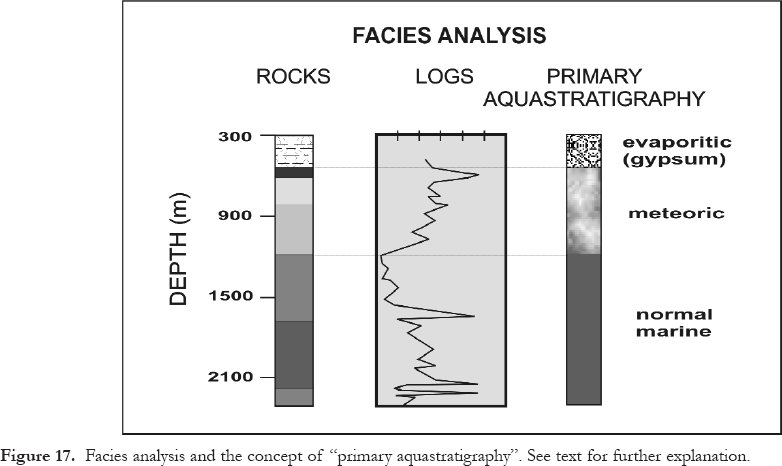
Display large image of Figure 17
80 Step 4: Establish burial curves, as deduced from stratigraphic, compaction, and/or thermal maturation data (Fig.20), and corresponding interpretation (in some cases educated speculation) of the paleohydrology (Fig. 21).
81 Step 5: Integrate with relevant extant data, especially standard petrophysical investigations (Lucia, 2000), if possible.
82 Step 6 (optional): Modeling, either forward or backward, of (a) facies (= primary porosity) parameters, such as from sequence stratigraphy (e.g., Tinker, 1996), (b) burial and thermal history (e.g., various studies reviewed in Hunt, 1996), (c) chemistry (e.g., Meshri and Ortoleva, 1991), as well as (d) present and past hydrology (England and Fleet, 1991; Rostron et al., 1997; Gvirtzman and Stanislavsky, 2000). In some modern studies, two or more types of modeling are undertaken in combination. However, as powerful as these types of modeling may be in certain cases, they are considered optional in the present context. Modeling is not necessary for most practical questions that a petroleum geologist is concerned with in a study of diagenesis.
83 Finally, it should be noted that the term "model" is used here in its true definition. It is necessary to emphasize this point because the term "model" is widely misused and generally subsituted for the term "interpretation". A model is not the same as an interpretation. Rather, a model is a complex concept that is based on a set of criteria, one of which is an interpretation: "a working hypothesis or precise simulation, by means of description, statistical data, or analogy, of a phenomenon or process that cannot be observed directly or that is difficult to observe directly. Models can be derived by various methods, e.g., by computer, from stereoscopic photographs, or from scaled experiments " (AGI, 1999). Walker (1992), using stratigraphic models as an example, elegantly summarized the general criteria of a model, which must act as: (1) a norm for purpose of comparison; (2) a framework and guide for future observations; (3) a predictor to new geological situations; and, (4) an integral basis for interpretation of the system it represents. Many studies have published so-called models that do not fulfill these criteria but are interpretations. Hence, Step 6 should not be mistaken for an interpretation of data.
Figure 18. Typical paragenetic sequence for Upper Devonian (D3) Leduc reefs in the central to southern parts of the Rimbey-Meadowbrook reef trend, Alberta, Canada. Based on Amthor et al. (1993), Machel et al. (1994), and Mountjoy et al. (1999).CONCLUSIONS
84 Porosity and permeability generally decrease with depth, but many sedimentary rocks, especially carbonates, have highly variable poroperm values that may be much higher or lower than the average. The various processes of porosity generation, preservation, and reduction "compete" with one another, and the present porosity and permeability characteristics represent the net result of all past processes. Considering the immense range of possibilities, generalized predictions of poroperm characteristics are risky, and each (carbonate) aquifer or reservoir unit should be investigated on an individual basis.
85 The methodology outlined in this article can be of great value in hydrocarbon exploration and development. Regarding the latter, the main value of diagenetic work is a better characterization of the pore and pore throat network that can be used to map or model a reservoir and its internal heterogeneities. This is especially useful in combination with petrophysical data, such as the behaviour under mercury injection. Regarding exploration, a characterization of diagenesis may help in predicting where, within a certain play type, the pools with the best porosity and permeability are located. This aspect arises out of an interpretation of paleofluid flow and the past fluid chemistry. Furthermore, many of the methods and the rationale discussed in this article can also be applied in surface geochemical exploration, which is based on leaking hydrocarbons creating distinctive diagenetic aureoles that are direct indicators for hydrocarbon pools at depth (e.g., Barker et al., 1991; Burton et al., 1993; Al-Shaieb et al., 1994; Machel, 1995; Tedesco, 1995; Schumacher and Abrams, 1996).
Figure 19. δ18 O/δ13 C-plot of Obed calcites and dolomites (see insert), compositional ranges of Late Devonian seawater calcites (SWC: brachiopods, marine cements), replacive matrix dolomites (MD) from Devonian carbonates in the Western Canada Sedimentary Basin, and calculated equilibrium values for Late Devonian seawater dolomites (SWD = dolomites formed in isotopic equilibrium from normal seawater at near-surface temperatures of 25°C). In combination with the petrographic data, this type of plot suggests that the matrix dolomites probably formed from chemically modified seawater at intermediate burial depths, and that the late calcites formed at much greater depths from hotter fluids that were charged with organic carbon from TSR. From Machel et al. (1996).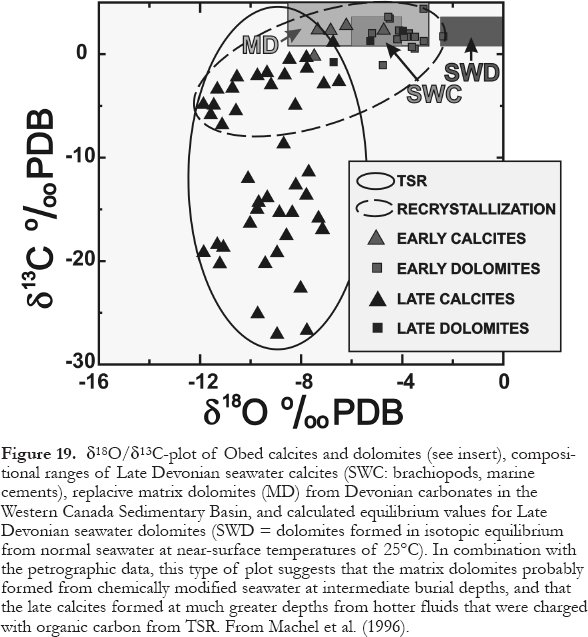
Display large image of Figure 19
ACKNOWLEDGEMENTS
The research leading up to the synthesis presented in this paper was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC). The constructive reviews by Ishan Al-Aasm, Dana S. Ulmer-Scholle and one anonymous reviewer are much appreciated.REFERENCES
AGI, 1999, Illustrated Dictionary of Earth Science: American Geological Institute. CD-ROM edition.
Al-Shaieb, Z., Cairns, J., and Puckette, J.,1994, Hydrocarbon-induced diagenetic aureoles (HDIA): indicators of deeper leaky reservoirs: Association of Petroleum Geochemical Explorationsists Bulletin, v. 10, p. 24-48.
Amthor, J.E., Mountjoy, E.W., and Machel, H.G., 1993, Subsurface dolomites in Upper Devonian Leduc Formation buildups, central part of Rimbey-Meadowbrook reef trend, Alberta, Canada: Bulletin of Canadian Petroleum Geology, v. 41, p. 164-185.
Amthor, J.E., Mountjoy, E.W., and Machel, H.G., 1994, Regional-scale porosity and permeability variations in Upper Devonian Leduc buildups: implications for reservoir development and prediction in carbonates: American Association Petroleum Geologists Bulletin, v. 78, p. 1541-1559.
Anderson, G.M. and Macqueen, R.W., 1982, Ore deposit models-6. Mississippi-Valley-Type lead-zinc deposits: Geoscience Canada, v. 9, p. 108-117.
Barker, C.E., Higley, D.K. and Dalziel, M.C., 1991, Using cathodoluminescence to map regionally zoned carbonate cements occurring in diagenetic aureoles above oil reservoirs: initial results from the Velma oil field, Oklahoma, in Barker, C.E. and Kopp, O.C., eds, Luminescence Microscopy and Spectroscopy -Qualitative and Quantitative Applications: SEPM (Society for Sedimentary Geology) Short Course, No. 25, p. 155-160.
Bathurst, R.G.C., 1987, Diagenetically enhanced bedding in argillaceous platform limestones: stratified cementation and selective compaction: Sedimentology,v. 34, p. 749-778.
Blatt, H., Middleton, G., and Murray, R., 1972, Origin of Sedimentary Rocks: Prentice Hall, 634 p.
Bodou, P., 1976, L'importance des joints stylolithiques dans la compaction des carbonates. Bulletin Centre Recherche Pau -SNPA, v. 10, p. 627-644.
Bonacci, O., 1987, Karst Hydrology: Springer Series in Physical Environment, 184 p.
Braithwaite, C. J. R. and Rizzi, G. 1997, The geometry and petrogenesis of hydrothermal dolomite at Navan, Ireland: Sedimentology, v. 44, p. 421-440.
Budd, D.A., 2001, Permeability loss with depth in the Cenozoic carbonate platform of west-central Florida: American Association Petroleum Geologists Bulletin, v. 85, p. 1253-1277.
Burton, E.A, Machel, H.G., and Qi, J., 1993, Thermodynamic constraints on anomalous magnetization in shallow and deep hydrocarbon seepage environments, in Aïssaoui, D.M., McNeill, D.F. and Hurley, N.F., eds., Applications of Paleomagnetism to Sedimentary Geology: SEPM (Society for Sedimentary Geology) Special Publication, No. 49, p. 193-207.
Choquette, P.W. and James, N.P., 1987, Diagenesis in limestones - 3. The deep burial environment: Geoscience Canada, v. 14, p. 3-35.
Choquette, P.W. and James, N.P., 1990, Limestones - The burial diagenetic environment, in McIlreath, I.E. and Morrow, D.A., eds., Diagenesis: Geoscience Canada Reprint Series 4, p. 75-112.
Choquette, P.W. and Pray, L.C., 1970, Geologic nomenclature and classification of porosity in sedimentary carbonates: American Association of Petroleum Geologists Bulletin, v. 54, p. 207-250.
Connan, J., 1984, Biodegradation of crude oils in reservoirs, in Brooks, J. and Welte, D., eds., Advances in Petroleum Geochemistry: Academic Press, London, v.1, p. 299-335.
Csoma, A.E. and Goldstein, R.H., 2004, Porosity reduction in meteoric-marine mixing zones: case studies illustrate some of the controls on calcite and aragonite cementation in mixing-zones. AAPG 2004 Annual Convention, Abstracts Volume, p. A29.
England, W.A. and Fleet, A.J. eds., 1991, Petroleum Migration: Geological Society, Special Publication No. 59, 280 p.
Fabricius, I.L., 2000, Interpretation of burial history and rebound from loading experiments and occurrence of microstylolites in mixed sediments of Caribbean Sites 999 and 1001, in Leckie, R.M., Sigurdson, H., Acton, G.D., and Draper, G. eds., Proceedings of the Ocean Drilling Program, Scientific Results, v. 165, p. 177-190.
Fagerstrom, J.A. and Weidlich, O., 1999, Strengths and weaknesses of the Reef Guild Concept and quantitative data: Application to the Upper Capitan-massive community (Permian), Guadeloupe Mountains, New Mexico, Texas: Facies, v. 40, p. 131-156.
Fletcher, R.C. and Merino, E., 2001, Mineral growth in rocks: kinetic-rheological models of replacement, vein formation, and syntectonic crystallization: Geochimica et Cosmochimica Acta, v. 65, p. 3733-3748.
Folk, R.L., 1965, Some aspects of recrystallization in ancient limestones, in Pray, L.C. and Murray, R.C., eds., Dolomitization and Limestones Diagenesis: SEPM Spec. Pub., No. 13, p. 14-48.
Galloway, W.E. and Hobday, D.K., 1983, Terrigenous Clastic Depositional Settings- Applications to Petroleum, Coal, and Uranium Exploration: Springer Verlag, 423 p.
Giles, M.R. and deBoer, R.B., 1989, Secondary porosity: creation of enhanced porosities in the subsurface from the dissolution of carbonate cements as a result of cooling formation waters: Marine and Petroleum Geology,v. 6, p. 261-269.
Giles, M.R. and Marshall, J.D., 1986, Constraints on the development of secondary porosity in the subsurface: reevaluation of processes: Marine and Petroleum Geology, v. 3, p. 243-255.
Gregg, J. M., Shelton, K. L., Johnson, A. W., Somerville, I. S., and Wright, W. R., 2001, Dolomitization of the Waulsortian Limestone (Lower Carboniferous) in the Irish Midlands: Sedimentology, v. 48, p. 745-766.
Gvirtzman, H. and Stanislavsky, E., 2000, Paleohydrology of hydrocarbon maturation, migration and accumulation in the Dead Sea Rift: Basin Research, v. 12, p. 79-93.
Halley, R.B. and Schmoker, J.W., 1983, High-porosity Cenozoic carbonate rocks of South Florida: progressive loss of porosity with depth: American Association of Petroleum Geologists Bulletin, v. 67, p. 191-200.
Hanor, J.S., 1987, Origin and migration of subsurface sedimentary brines: Society of Economic Paleontologists and Mineralogists Short Course, No. 21, 247 p.
Hanor, J.S., 1994, Origin of saline fluids in sedimentary basins, in Parnell, J., ed., Geofluids - Origin, Migration, and Evolution of Fluids in Sedimentary Basins: Geological Society America Special Publication, No. 78, p. 151-174.
Harris, P.M. and Kowalik, W.S. eds., 1994, Satellite Images of Carbonate Depositional Settings: AAPG Methods in Exploration Series, v. 11, 147 p.
Heydari, E., 1997, Hydrotectonic models of burial diagenesis in platform carbonates based on formation water geochemistry in North American sedimentary basins, in Montañez, I.P., Gregg, J.M. and Shelton, K.L., eds., Basin-wide Diagenetic Patterns: Integrated Petrologic, Geochemical, and Hydrologic Considerations: SEPM Special Publication, No. 57, p. 53-79.
Hunt, J.M., 1996, Petroleum Geochemistry and Geology. Second Edition: W.H. Freeman and Company, NY, 743 p.
Hutcheon, I., 1992, Subsurface dissolution porosity in carbonates - recognition, causes and implications. Part 2: AAPG/CSPG Short Course Notes, 20 p.
James, N.P., and Choquette, P.W. 1984, Diagenesis 9. Limestones - the meteoric diagenetic environment: Geoscience Canada, v. 11, p. 161-194.
James, N.P., and Choquette, P.W., eds., 1987, Paleokarst: Springer Verlag, 416 p.
James, N.P., and Choquette, P.W., 1990, Limestones - the sea floor diagenetic environment: Geoscience Canada Reprint Series 4, p. 13-34.
Krouse, H.R., 1977, Sulfur isotope studies and their role in petroleum exploration: Journal Geochemical Exploration, v. 7, p. 189-211.
Land, L. S. 1985, The origin of massive dolomite: Journal of Geological Education, v. 33, p. 112-125.
Land, L.S., 1997, Mass transfer during burial diagenesis in the Gulf of Mexico sedimentary basin: an overview, in Montañez, I.P., Gregg, J.M. and Shelton, K.L., eds., Basin-wide Diagenetic Patterns: Integrated Petrologic, Geochemical, and Hydrologic Considerations: SEPM Special Publication, No. 57, p. 29-39.
Laudon, R.C., 1996, Principles of Petroleum Development Geology: Prentice Hall,NJ, 267 p.
Lee, M-K, 1997, Predicting diagenetic effects of groundwater flow in sedimentary basins: a modeling approach with examples, in Montañez, I.P., Gregg, J.M. and Shelton, K.L., eds., Basin-wide Diagenetic Patterns: Integrated Petrologic, Geochemical, and Hydrologic Considerations: SEPM Special Publication, No. 57, p. 3-14.
Levine, J.R., Samson, I.M. and Hesse, R.,1991, Occurrence of fracture-hosted impsonite and petroleum fluid inclusions, Quebec City Region, Canada: American Association of Petroleum Geologists Bulletin, v. 75, p. 139-155.
Lind, I.L., 1993, Stylolites in chalk from Leg 130, Ontong Java Plateau, in Berger, W.H., Kroenke, J.W. and Mayer, L.A., eds., Proceedings of the Ocean Drilling Program, Scientific Results, v. 130, p. 445-451.
Lovley, D.R. and Chapelle, F.H., 1995, Deep subsurface microbial processes: Reviews of Geophysics, v. 33, p. 365-381.
Lucia, F.J., 2000, Carbonate Reservoir Characterization: Springer-Verlag, 226 p.
Lucia, F.J., 2002, Origins and petrophysics of dolostone pore space, in Rizzi, G., Darke, G. and Braithwaite, C. (convenors), The geometry and petrogenesis of dolomite hydrocarbon reservoirs, Final Programme and Abstracts: Geological Society Petroleum Group, Burlington House - Picadilly, London, 3-4th December 2002, 1 p.
Lucia, F.J., 2004, Origin and petrophysics of dolostone pore space, in Braithwaite, C.J.R., Rizzi, G., and Darke, G. eds., The Geometry and Petrogenesis of Dolomite Hydrocarbon Reservoirs. Geological Society, London, Special Publication, No. 235, p. 141-155.
Lucia, F.J., and Major, R.P., 1994, Porosity evolution through hypersaline reflux dolomitization, in, Purser, B., Tucker. M., and Zenger, D., eds., Dolomites - A volume in honour of Dolomieu: Special Publication 21, International Association of Sedimentologists, p. 325-341.
Lucia, F.J., Jones, R.H., and Jennings, J.W., 2004, Poikilotopic anhydrite enhances reservoir quality: AAPG 2004 Annual Convention, Abstracts Volume, p. A88.
Luo, P. and Machel, H.G., 1995, Pore size and pore-throat types in a heterogeneous dolostone reservoir, Devonian Grosmont Formation, Western Canada Sedimentary Basin: American Association of Petroleum Geologists Bulletin, v. 79, p. 1698-1720.
Luo, P., Machel, H.G., and Shaw, J., 1994, Petrophysical properties of matrix blocks of a heterogeneous dolostone reservoir -the Upper Devonian Grosmont Formation, Alberta, Canada: Bulletin of Canadian Petroleum Geology, v. 42, p. 465-481.
Machel, H.G., 1985, Fibrous gypsum and fibrous anhydrite in vein: Sedimentology,v. 32, p. 443- 454.
Machel, H.G., 1987, Some aspects of diagenetic sulphate-hydrocarbon redox-reactions, in Marshall, J.D. ed., Diagenesis of Sedimentary Sequences: Geological Society, London, Special Publication, No. 36, p. 15-28.
Machel, H.G., 1995, Magnetic mineral assemblages and magnetic contrasts in diagenetic environments - with implications for studies of paleomagnetism, hydrocarbon migration and exploration, in Turner, P. and Turner, A., eds., Paleomagnetic Applications in Hydrocarbon Exploration and Production: Geological Society, London, Special Publication, No. 98, p. 9-29.
Machel, H.G.,1997, Recrystallization versus neomorphism, and the concept of ‘ significant recrystallization' in dolomite research: Sedimentary Geology, v. 113, p. 161-168.
Machel, H.G., 1998, Gas souring by thermochemical sulfate reduction at 140℃: Discussion. American Association of Petroleum Geologists Bulletin, v. 82, p. 1870-1873.
Machel, H.G., 1999, Effects of groundwater flow on mineral diagenesis, with emphasis on carbonate aquifers: Hydrogeology Journal, v. 7, p. 94-107.
Machel, H.G., 2001, Bacterial and thermochemical sulfate reduction in diagenetic setting: Sedimentary Geology, v. 140, p. 143-175.
Machel, H.G., 2004, Concepts and models of dolomitization: a critical reappraisal, in Braithwaite, C.J.R., Rizzi, G., and Darke,G. eds., The Geometry and Petrogenesis of Dolomite Hydrocarbon Reservoirs: Geological Society, London, Special Publication, No. 235, p. 7-63.
Machel, H.G., and Cavell, P.A., 1999, Low-flux, tectonically induced squeegee fluid flow ("hot flash") into the Rocky Mountain Foreland Basin: Bulletin Canadian Petroleum Geology, v. 47, p. 510-533.
Machel, H.G. and Foght, J., 2000, Products and depth limits of microbial activity in petroliferous subsurface settings in Riding, R.E. and Awramik, S.M. eds., Microbial Sediments: Springer Verlag, p. 105-120.
Machel, H.G. and Huebscher, H., 2000, The Devonian Grosmont heavy oil reservoir in Alberta, Canada:. Zentralblatt für Geologie und Paläontologie, Teil I, Heft 1/2, p. 55-84.
Machel, H.G. and Hunter, I.G., 1994, Facies models for Middle to Late Devonian shallow-marine carbonates, with comparisons to modern reefs – a guide for facies analysis: Facies, v. 30, p. 155-176.
Machel, H.G., and Lonnee, J., 2002, Hydrothermal dolomite - a product of poor definition and imagination: Sedimentary Geology, v. 152, p. 163-171.
Machel, H.G. and Mountjoy, E.W., 1986, Chemistry and environments of dolomitization - a reappraisal: Earth Science Reviews, v. 23, p. 175-222.
Machel, H.G., and Mountjoy, E.W., 1990, Coastal mixing zone dolomite, forward modeling, and massive dolomitization of platform-margin carbonates -Discussion: Journal of Sedimentary Petrology, v. 60, p. 1008-1012.
Machel, H.G., Mountjoy, E.W., and Amthor, J.E., 1994, Dolomitisierung von devonis-chen Riff- und Plattformkarbonaten in West-Kanada. Zentralblatt für Geologie und Paläontologie, Teil I, (7/8), p. 941-957.
Machel, H.G., Krouse, H.R., and Sassen, R., 1995a, Products and distinguishing criteria of bacterial and thermochemical sulfate reduction: Applied Geochemistry, v. 10, p. 373-389.
Machel, H.G., Krouse, H.R., Riciputi, L.R. and Cole, D.R., 1995b, Devonian Nisku sour gas play, Canada: A unique natural laboratory for study of thermochemical sulfate reduction, in Vairavamurthy, M.A. and Schoonen, M.A.A. eds., Geochemical Transformations of Sedimentary Sulfur: ACS Symposium Series, No. 612, p. 439-454.
Machel, H.G., Cavell, P.A. and Patey, K.S., 1996, Isotopic evidence for carbonate cementation and recrystallization, and for tectonic expulsion of fluids into the Western Canada Sedimentary Basin: Geological Society of America Bulletin,v. 108, p. 1108-1119.
Machel, H.G., Buschkuehle, B.E. and Michael, K., 2001, Squeegee flow in Devonian carbonate aquifers in Alberta, Canada, in Cidu, R. ed., Water-rock interaction, Vol. 1. Proceedings of the Tenth International Symposium on Water-Rock-Interaction WRI-10, Villasimius, Italy, p. 631-634.
Maliva, R.G. and Dickson, J.A.D., 1992, Microfacies and diagenetic controls of porosity in Cretaceous/ Tertiary chalks, Ekofisk Field, Norwegian North Sea: American Association Petroleum Geologists Bulletin, v. 76, p. 1825-1838.
Marquez, X. and Mountjoy, E.W., 1996, Origin of microfractures in the Upper Devonian Leduc Strachan reservoir, deep Alberta Basin: American Association of Petroleum Geologist, Bulletin, v. 80, p. 570-588
Meshri, I.D. and Ortoleva, P.J. eds., 1990, Prediction of reservoir quality through chemical modelling: American Association of Petroleum Geology, Memoir, No. 49. 175 p
Montañez, I.P., 1997, Secondary porosity and late diagenetic cements in the Upper Knox Group, central Tennessee region: a temporal and spatial history of fluid flow conduit development within the Knox regional aquifer, in Montañez, I.P., Gregg, J.M. and Shelton, K.L., eds., Basin-wide Diagenetic Patterns: Integrated Petrologic, Geochemical, and Hydrologic Considerations: SEPM Special Publication, No. 57, p. 101-117.
Montañez, I.P., Gregg, J.M. and Shelton, K.L., 1997, eds., Basin-wide Diagenetic Patterns: Integrated Petrologic, Geochemical, and Hydrologic Considerations: SEPM Special Publication, No. 57, 302 p.
Moore, G.E. and Wilde, G.L. 1986, eds., Lower Middle Guadalupian Facies, Stratigraphy, and Reservoir Geometries, San Andres/Grayburg Formations. Guadalupe Mountains, New Mexico and Texas: Permian Basin Section of SEPM, Publication No. 86-25, 144 p.
Morse, J.W. and Casey, W.H., 1988, Ostwald processes and mineral paragenesis in sediments. American Journal of Science, v. 288, p. 537-560.
Morse, J.W., Hanor, J.S., and He, S., 1997, The role of mixing and migration of basinal waters in carbonate mineral mass transport, in Montañez, I.P., Gregg, J.M. and Shelton, K.L., eds., Basin-wide Diagenetic Patterns: Integrated Petrologic, Geochemical, and Hydrologic Considerations: SEPM Special Publication, No. 57, p. 41-50.
Mountjoy, E.W., Whittaker, S., Williams-Jones, A.E., Qing, H., Drivet, E., and Marquez, E., 1997, Variable fluid and heat flow regimes in three Devonian dolomite conduit systems, Western Canada Sedimentary Basins: isotopic and fluid inclusion evidence/constraints, in Montañez, I.P., Gregg, J.M. and Shelton, K.L., eds., Basin-wide Diagenetic Patterns: Integrated Petrologic, Geochemical, and Hydrologic Considerations: SEPM Special Publication, No. 57, p. 119-137.
Mountjoy, E.W., Machel, H.G., Green, D. Duggan, J., and Williams-Jones, A.E., 1999, Devonian matrix dolomites and deep burial carbonate cements: a comparison between the Rimbey-Meadowbrook reef trend and the deep basin of west-central Alberta: Bulletin Canadian Petroleum Geology, v. 47, p. 487-509.
Munnecke, A., Westphal, H., Elrick, M., and Reijmer, J.J.G., 2001, The mineralogical composition of precursor sediments of calcacrous rhythmites: a new approach: International Journal of Earth Sciences, v. 90, p. 795-812.
Nesbitt, B. and Muehlenbachs, K., 1997, Paleo-hydrogeology of Late Proterozoic units of southeastern Canadian Cordillera: American Journal of Science,v. 297, p. 359-392.
Nicholson, A.D., 1992, Thermochemical sulfate reduction in the Whitney Canyon -Crater Creek Field, Wyoming: Unpublished PhD Thesis, Colorado School of Mines, 241 pp.
Pedersen, T. and Bjorlykke, K., 1994, Fluid flow in sedimentary basins: model of pore water flow in vertical fracture: Basin Research, v. 6, p. 1-16.
Plummer, L.N., 1975, Mixing of seawater with calcium carbonate groundwater: Geological Society America Memoir, No. 142, p. 219-238.
Prezbindowski, D.R., 1985, Burial cementation - is it important? A case study, Stuart City Trend, South Central Texas, inSchneidermann, N. and Harris, P.M., eds., Carbonate Cements: SEPM Special Publication, No. 36, p. 241-264.
Purser, B., Tucker M., and Zenger, D. eds., 1994, Dolomites - A volume in honour of Dolomieu. Special Publication Number 21, International Association of Sedimentologists, 395 p.
Ramsey, J.G, 1980, The crack-seal mechanism of rock deformation: Nature, v. 284, p. 135-139.
Ricken, W., 1987, The carbonate compaction law: a new tool: Sedimentology, v. 34, p. 571-583.
Rostron, B.J., Tóth, J. and Machel, H.G., 1997, Fluid flow, hydrochemistry, and petroleum entrapment in Devonian reef complexes, south-central Alberta, Canada, in Montañez, I.P., Gregg, J.M. and Shelton, K.L. eds., Basin-wide Diagenetic Patterns: Integrated Petrologic, Geochemical, and Hydrologic Considerations: SEPM Special Publication, No. 57, p. 139-155.
Robie, R.A., Hemingway, B.S., and Fisher, J.R., 1979, Thermodynamic properties of mineral and related substances at 298.15 K and 1 Bar pressure and at higher pressures: Geological Survey Bulletin 1452, 456 p..
Schmidt, V. and McDonald, D.A., 1979, The role of secondary porosity in the course of sandstone diagenesis: SEPM, Special Publication No. 26, p. 175-207.
Schmoker, J.W. and Halley, R.B., 1982, Carbonate porosity versus depth: a predictable relation for south Florida: American Association Petroleum Geologists Bulletin, v. 66, p. 2561-2570.
Schmoker, J.W., Krystinik, K.B., and Halley, RR.B., 1985, Selected characteristics of limestone and dolomite reservoirs in the United States: American Association Petroleum Geologists Bulletin, v. 69, p. 733-741.
Schneidermann, N. and Harris, P.M., eds.,1985, Carbonate Cements: SEPM Special Publication No. 36, 379 p.
Schoell, M., 1984, Recent advances in petroleum geochemistry: Organic Geochemistry, v. 6, p. 645-663.
Scholle, P.A. and Halley, R.B., 1985, Burial diagenesis: out of sight, out of mind! in Schneidermann, N. and Harris, P.M., eds., Carbonate Cements: SEPM Special Publication, No. 36, p. 309-334.
Schroeder, J.H., and Purser, B.H., eds., 1986, Reef Diagenesis: Springer Verlag, 455 p.
Schroyen, K. and Muchez, P., 1998, Paleofluid flow along Variscian thrust faults: the role and importance of metamorphism, in Cañaveras, J.C., Ángeles García del Cura, M., and Soria, J., eds., 15th International Sedimentological Congress, April 12-17, 1998, Alicante, Spain, Abstracts, p. 705-706.
Schumacher, D. and Abrams, M.A., eds., 1996, Hydrocarbon migration and its near-surface expression: American Association of Petroleum Geology Memoir, No. 66, 446 p.
Simpson, G.P., 1999, Sulfate reduction and fluid chemistry of the Devonian Leduc and Nisku Formations in south-central Alberta: Unpublished. Ph.D. Thesis, University of Calgary, 228 p.
Simpson, G., Yang, C., and Hutcheon, I., 1996, Thermochemical sulfate reduction: a local process that does not generate thermal anomalies, in G.M. Ross (compiler), 1995 Alberta Basement Transects Workshop, Lithoprobe Report # 51. Lithoprobe Secretariat, University of British Columbia, p. 241-245.
Smart, P.L., Dawans, J.M. and Whitaker, F. 1988, Carbonate dissolution in a modern mixing zone, South Andros, Bahamas: Nature, v. 355, p. 811-813.
Stoute, E.L. and Harris, P.M., eds., 1995, Hydrocarbon reservoir characterization -Geologic framework and flow unit modeling: SEPM Short Course No. 34, 357 p.
Surdam, R.C., Dunn, T.L., Heasler, H.P. and MacGowan, D.B., 1989, Porosity evolution in sandstone/shale systems, in Hutcheon, I., ed., Short Course on Burial Diagenesis: Mineralogical Association of Canada, p. 61-134.
Tedesco, S.A., 1995, Surface Geochemistryin Petroleum Exploration: Chapman and Hall, 206 p.
Tinker, S.W., 1996, Building the 3-D jigsaw puzzle: applications of sequence stratigraphy to 3-D reservoir characterization: American Association of Petroleum Geology Bulletin, v. 80, p. 460-485.
Tóth, J., 1963, A theoretical analysis of groundwater flow in small drainage basins: Journal Geophysical Research, v. 68, p. 4795-4812.
Tóth, J. and Otto, C.J., 1994, Hydrogeology and oil deposits at Pechelbronn-Soultz -Upper Rhine Graben: Acta Geological Hungarica, v. 36, no. 4, p. 375-393.
Trurnit, P. and Amstutz, G.C., 1979, Die Bedeutung des Rueckstandes von Druck-Loesungsvorgaengen fuer stratigraphische Abfolgen, Wechsellagerung and Lagerstaettenbildung: Jahrestagung Geologische Vereinigung, Band 68, Heft 3, p. 1107-1124.
Usdowski, E., 1994, Synthesis of dolomite and geochemical implications, in Purser, B., Tucker M., and Zenger, D., eds., Dolomites - A volume in honour of Dolomieu: Special Publication Number 21, International Association of Sedimentologists, p. 345-360.
Van Tuyl, F.M., 1914, The origin of dolomite: Annual Report 1914, Iowa Geological Survey, Vol XXV, p. 257-421.
Volkman, J.K., Alexander, R., Kagi, R.I., and Woodhouse, 1983, Demethylated hopanes in crude oils and their applications in petroleum geochemistry: Geochimica Cosmochimica Acta, v. 47,p. 785-794.
Walker, R.G., 1992, Facies, facies models and modern stratigraphic concepts, in Walker, R.G. and James, N.P., eds., Facies Models - Response to Sea Level Change: Geological Association of Canada, p. 1-14.
Walls, R.A. and Burrowes G., 1985, The role of cementation in the diagenetic history of Devonian reefs, Western Canada, in Schneidermann, N. and Harris, P.M.,eds., Carbonate Cements: SEPM Special Publication, No. 36, p. 185-220.
Walter, L.M., 1985, Relative reactivity of skeletal carbonate during dissolution: implications for diagenesis, in Schneidermann, N. and Harris, P.M., eds., Carbonate Cements: SEPM Special Publication, No. 36, p. 3-16.
Weidlich O., Bernecker, M. and Fluegel E., 1993, Combined Quantitative Analysis and Microfacies Studies of Ancient Reefs: An integrated Approach to Upper Permian and Upper Triassic Reef Carbonates (Sultanate of Oman): Facies,v. 28, p. 115-144.
Wiltschko, D.V. and Morse, J.W., 2001, Crystallization pressure versus "crack-seal" as the mechansim for banded veins: Geology, v. 29, p. 79-83.
Winkler, H.G.F., 1979, Petrogenesis of Metamorphic Rocks. 5th edition: Springer Verlag, 348 p..
Woody, R.E., Gregg, J.M. and Koederitz, L.F., 1996, Effect of texture on petro-physical properties of dolomite: evidence from the Cambrian-Ordovician of southeastern Missouri: American Association of Petroleum Geology Bulletin, v. 80, p. 119-132.
Woronik, R.E. and Land, L.S., 1985, Late burial diagenesis, Lower Cretaceous Pearsall and Lower Glen Rose Formations, South Texas, in Schneidermann, N. and Harris, P.M., eds., Carbonate Cements: SEPM Special Publication, No. 36, p. 265-275.