Articles
Shining Light on Black Rock Coatings in Smelter-Impacted Areas
SUMMARY
Earth scientists have long known of the existence of black coatings on exposed rocks in smelter-impacted areas such as Sudbury, Ontario or Rouyn-Noranda, Québec. Black rock coatings in the Greater Sudbury area are remarkable geological records of atmospheric conditions, including mixing, scavenging, and oxidation processes, deposition rates, and the nature and source of anthropogenic releases to the atmosphere. The coatings are composed of an amorphous silica matrix that has trapped atmosphere-borne nanoparticles and has preserved their chemical and isotopic signature. These coatings are the product of high emissions of SO2 and subsequent non-stoichiometric dissolution of exposed siliceous rocks. The coatings contain spherical smelter-derived Cu–Ni-oxide particulate matter (micrometre and nanometre-sized) and metal-sulphate-rich layers composed of nanometre aggregates of Fe–Cu sulphates. Lead, As, and Se-bearing nanoparticles emitted from smelters are incorporated in metal-sulphate-rich layers along the atmosphere-coating interface, presumably during coating formation. On a regional scale, ratios between different metal(loid)s in the coatings indicate that small diameter primary Pb, As and Se-bearing sulphate aerosols have been deposited at higher rates compared to larger, Ni-bearing particulate matter. High sulphur isotope values in coatings closer to smelting centres and their decrease with distance from the smelters is attributed to an increase in mixing of primary and secondary sulphates.
SOMMAIRE
Les géoscientifiques connaissent depuis longtemps l’existence d’une couche noire sur les roches exposées aux abords des fonderies comme celles de Sud-bury en Ontario ou Rouyn-Noranda au Québec. Les couches noires des roches de la grande région de Sudbury constituent de remarquables enregistrements géologiques des phénomènes atmosphériques, notamment des processus de mélange, de piégeage, et d'oxydation, ainsi que des taux de sédimentation et de la nature et de l’origine des rejets anthropiques dans l'atmosphère. Ces couches noires sont constituées d'une matrice de silice amorphe qui a piégé des nanoparticules atmosphériques et conservé leur signature chimique et isotopique. Ces couches noires sont le produit de fortes émissions atmosphériques de SO2 et d’une dissolution non-stœchiométrique subséquente des roches siliceuses exposées. Ces couches noires contiennent des sphérules de particules atmosphériques d’oxydes de Cu-Ni (de taille micrométrique et nanométrique) issues de la fonderie, et des couches riches en sulfate de métaux constituées d’agrégats nanométriques de sulfates de Fe-Cu. Les nanoparticules de plomb, d’As et de Se émises par les fonderies sont incorporées dans les couches riches en sulfate de métal à l'interface de l’atmosphère et de cette couche, probablement lors de la formation de cette couche. À l’échelle régionale, les rapports de concentration des différents métaux ou métalloïdes dans les couches noires indiquent que les aérosols de faible diamètre de sulfate de Pb, d’As et de Se primaires ont été déposés à des taux plus élevés que les particules nickélifères de plus grande dimension. Les valeurs plus élevées des isotopes du soufre observées dans les couches à proximité des fonderies et leur diminution en fonction de l’éloignement des fonderies sont attribuées à une augmentation du mélange entre sulfates à l’émission et post-émission.
INTRODUCTION
1 The Greater Sudbury area in Ontario, Canada, is one of the major base-metal smelting regions in North America. Three major Cu–Ni smelters (Copper Cliff, Coniston and Falconbridge; Fig. 1a) operated in Sudbury during the last century and annually emitted over 2 million tons of SO2 and over 1000 tons of Cu, Ni, Pb and As during peak years of emissions. Logging of area forests prior to, and during, the mining activities, in conjunction with emissions of SO2 and metal(loid)s, resulted in loss of vegetation in the Greater Sudbury area and development of large areas of barren rock and soil. These conditions yielded a landscape characterized by thin, acidic soils and black rock coatings (Gundermann and Hutchinson 1995). The black rock coatings of the Greater Sudbury area (Fig. 1b), which have been observed since the first half of the 20th century, are the product of atmosphere–rock interactions on exposed surfaces and infill fractures (Mantha et al. 2012a, b).Inorganic rock coatings occur around the world (Dorn 1998); thick coatings are commonly observed in deserts (Fisk 1971; Smith and Whalley 1988), volcanic weathering environments (Curtiss et al. 1985; Minitti et al. 2007; Schindler et al. 2010), mine tailings (Schindler et al. 2009; Durocher and Schindler 2011), Antarctica (Friedmann and Weed 1987; Weed and Norton 1991; Giorgetti and Baroni 2007) and glaciated environments (Whalley et al. 1990). They contain a variety of environmental products, such as dust particulate matter, in situ-formed minerals, complex organic molecules and micro-fossils (Langworthy et al. 2010).
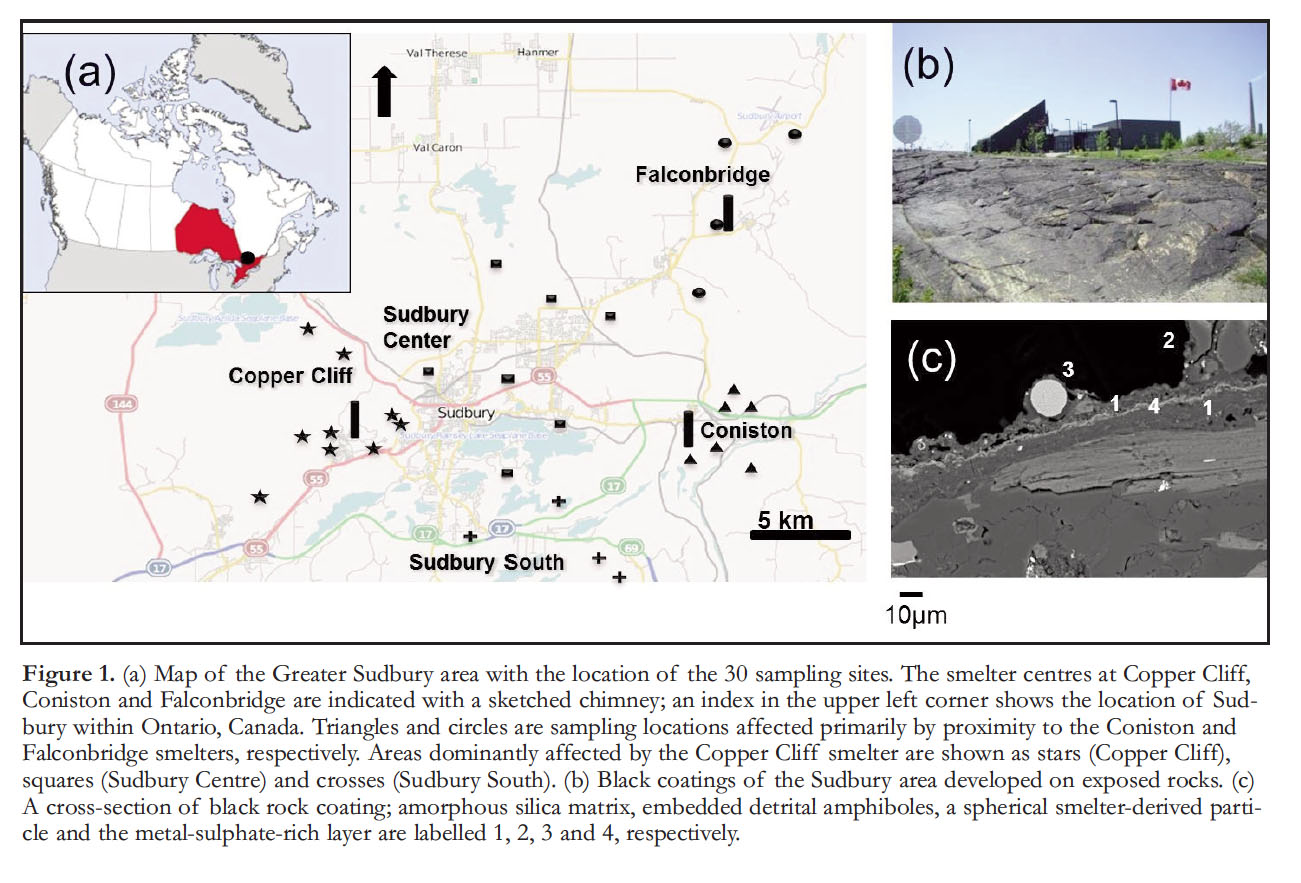 Display large image of Figure 1
Display large image of Figure 12 Surface coatings on rocks and building stones are useful forensic tools for monitoring recent and past air pollutants and tracing their sources. Coatings formed in deserts, in proximity to smelters and in highly polluted urban environments, may capture common pollutants such as metal-bearing particulate matter, sulphate aerosols and radionuclides during their formation (Nord et al. 1994; Hodge et al. 2005; Wayne et al. 2006; Smith and Prikryl 2007; Hoar et al. 2011; Mantha et al. 2012a, b). For example, rock varnishes collected around the Nevada Test Site and near power plants in Nevada contain anomalously low 240Pu/239Pu mass ratios in comparison to observed ratios worldwide (Hoar et al. 2011). Gypsum coatings on building stones in Prague record a change in the sulphur isotope composition from the centre to the periphery of the city, which was initially explained by a sulphur-isotope (S-isotope) fractionation process (Buzek and Šrámek 1985), but has been recently interpreted as a mixing process of primary and secondary sulphate aerosols (Mantha et al. 2012b).
3 The forensic examination of rock coatings is also valuable in providing an understanding of past terrestrial or planetary environments (e.g. on Mars; McLennan 2003; Milliken et al. 2008; Squyres et al. 2008), because coatings may preserve particulate matter and mineral phases, containing information about past climatic conditions, volcanic events, and biological activity.
4 The areas surrounding base-metal smelters are ideal locations for the study of past atmospheric particulate matter (including aerosols) because the release of vast quantities of SO2 resulted in increased chemical weathering and ideal conditions for the formation of thick coatings on exposed rock surfaces. The chemical, mineralogical and isotopic composition of these coatings can be compared with historical data on metal emissions, metal speciation in smelter plumes, the isotopic composition of the smelted ore and smelter gases, and metal concentrations in the surrounding soils. The known point sources of SO2 and metal particulate matter, as well as the available historical records of local emissions, represent clear advantages in the study of coatings compared to studies of coatings proximal to volcanoes (e.g. Schiffmann et al. 2006), in deserts (e.g. Perry et al. 2006) and on Antarctica (Giorgetti and Baroni 2007), which are all affected by various sources, inputs and processes potentially on a global scale.
5 This paper demonstrates that silica coatings in the vicinity of base-metal smelters record the mineralogical, chemical and isotopic signature of atmospheric nano- to micro-size particulate matter, as well as changes in the proportion of secondary and primary aerosols with distance from the smelter point-source.
MATERIAL AND METHODS
6 Black rock coatings were sampled from 30 different locations within the Greater Sudbury area (Fig. 1a), focusing on regions in the vicinity of the smelters and other areas affected by smelting emissions because of their location with respect to the dominant northeasterly wind direction. Coatings were examined using scanning electron microscopy (JEOL 6405), electron microprobe (Cameca SX-100), powder X-ray diffraction (Philips PW 1729), scanning transmission electron microscope (FEI Titan 80-300), micro- X-ray fluorescence (VESPERS beamline, Canadian Lightsource), and laser ablation–inductively coupled plasma mass spectrometry (LA-ICP-MS; Thermo-Fisher X II Series mass spectrometer; details in Mantha et al. 2012a).
7 Twenty-six samples were prepared for sulphur-isotope measurements by collecting scrapings of the coatings. The δ34S values of the samples were analyzed using a MAT 252 Stable Isotope Ratio Mass Spectrometer coupled with a Carlo Erba NCS 2500 Elemental Analyzer at the Queen’s University Facility for Isotope Research. The δ34S values of the coating material were calculated by normalizing the 34S/32S ratios in the sample to the same ratio in the Canyon Diablo Troilite (CDT) standard, and are expressed as δ values in units of permil. The scrapings also contained material from the underlying rock; however, optical microscope and scanning electron microscope (SEM) examination indicated the absence of sulphur-bearing minerals in the underlying rock (Mantha et al. 2012b).
RESULTS AND DISCUSSIONS
8 Black coatings occur on weathered granites, gneisses, metabasalts and gabbros throughout the Sudbury area (Mantha et al. 2012a; Fig. 1b). Their distribution within the area is consistent with a smelter impacted zone, in terms of SO2 emissions, soil degradation and vegetation damage. The distribution of metals from a point source and subsequent environmental damage is consistently shown to be controlled, in part, by prevailing winds (Knight and Henderson 2006).
Mineralogical and Chemical Composition
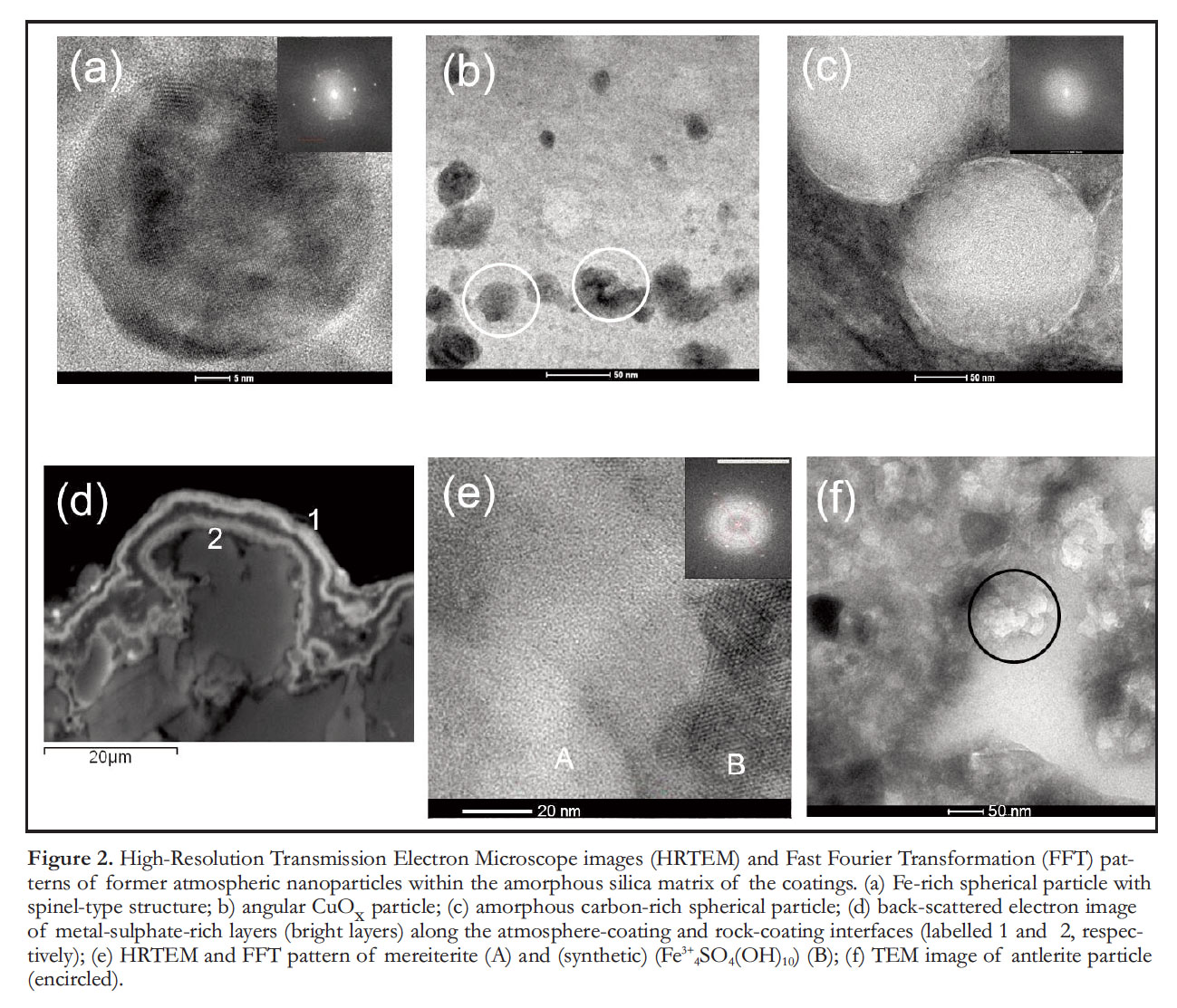
9 Coating thickness varies from <5 µm to >100 µm and was observed to reach up to 200 µm including accumulations of particulate matter and detrital mineral grains. The rock-coating boundary with the underlying rock is typically sharp at the micrometre scale. Feldspars and amphiboles in the underlying rock are weathered and the interstices between these minerals show a loss of mass and occasional infilling of cracks by coating material. Coatings are composed of a porous amorphous silica matrix that contains a wide variety of detrital and smelter-derived particulate matter as well as metal sulphate-rich layers (Fig. 1c). Detrital particles originate from the underlying rock or from adjacent rocks and soils and are composed of micrometre-size feldspar, amphibole and quartz fragments (Fig. 1c) as well as nanoparticles of hematite, chlorite, and montmorillonite. Various types of smelter-derived nanoparticles occur within the silica matrix. There are spherical, spinel-type particulate matter (mainly magnetite (Fe3O4), cupro spinel (CuFe2O4), and trevorite (NiFe2O4); Fig. 2a), as well as angular CuO x nanoparticles (Fig. 2b) and amorphous carbon nanoparticles (Fig. 2c). The latter are similar in appearance to coke particulate matter observed in soils surrounding a Zn-smelter in France (Sivry et al. 2010), and are commonly generated during the burning of coal for metallurgical purposes. Smelter-derived particular matter are mainly composed of magnetite and fayalite (Fe2SiO4), and are locally observed with diameters greater than the average thickness of coatings in which they are embedded (Mantha et al. 2012a; Fig. 1c).
10 Metal-sulphate-rich layers are prominent features within the silica-rich matrix (Figs. 1c and 2d). They contain high concentrations of metal(loid)s such as Pb (max. 10 wt%), Cu (max. 2.2 wt%), As (max. 1.8 wt%) and Se (max. 0.1 wt%) and are composed of nanometre aggregates of numerous sulphates such as (synthetic) (Fe3+4SO4(OH)10), goldichite (KFe3+(SO4)2(H2O)4), mereiterite (K2Fe2+(SO4)2(H2O)4), guildite (CuFe3+(SO4)2(OH)(H2O)4), butlerite (Fe3+(SO4)(OH)(H2O)2) and antlerite (Cu3(SO4)(OH)4) (Fig. 2e, f).
Model for Coatings Formation
11 Acidic emissions and wet precipitation, the result of vast quantities of SO 2 released from smelting, increased the chemical weathering rate of exposed rocks in the Greater Sudbury area. Non-stoichiometric dissolution of silicate minerals at the rock surface resulted in the formation of a hydrous silica gel having a lower viscosity than other solid-state weathering products. This allowed the trapping and accumulation of particulate matter (e.g. Perry et al. 2006), best observed in Figure 1c, in which a thin silica-rich layer holds a spherical particle with a significantly larger diameter. The silica gel also contained a connected interstitial pore system that allowed the mixing of pore fluids, the in situ growth of minerals, and the entombment of particular matter. In these pore systems, dissolution of trapped nanoparticles by sulphuric acid produced metal(loid)-sulphate-rich pore solutions, which subsequently mixed with solutions containing elements released via the dissolution of the underlying rock (e.g. Na, K, Al, Fe). This mixing process resulted in the precipitation of K–Fe-sulphates on the atmosphere-coating and rock-coating interfaces (Mantha et al. 2012a; Fig. 2d).
 Display large image of Figure 2
Display large image of Figure 212 Adsorbed silica species have the ability to modify transformation processes of minerals (Cornell and Schwertmann 1996). For example, adsorbed silica species are known to slow down or even inhibit the transformation of ferrihydrite (Fe-hydroxide) and jarosite (Fe-sulphate) into the thermodynamically more stable phases, goethite and hematite. Hence, dissolved silica-species sorbed to precipitated and encapsulated precipitates of goldichite, mereiterite, guildite, butlerite and antlerite may have inhibited their transformation to thermodynamically more stable minerals. As the gel-like material further condensed and dehydrated, it began to lose its gel properties, resulting in the limited diffusion of dissolved substances (including H+) and effectively protecting nucleated and incorporated minerals. The apparent disequilibrium between many embedded nanoparticles such as clays and (hydr)oxides (stable at near neutral pH) and the Fe-Cu-sulphates (stable at low pH) indicates that prior to the hardening of the gel, trapped nanoparticles were encapsulated in pores, which prevented their equilibration with acidic metal-sulphate-bearing fluids.
13 This model differs from previous models for the formation of metal-sulphate- and silica-enriched coatings (Schiffman et al. 2006; Minitti et al. 2007) because it emphasizes theinitial formation of a hydrous silica gel-type layer and proposes that all subsequent processes (trapping of particulate matter, their dissolution and encapsulation) are controlled by the properties of this silica layer.
Change in Chemical Composition on the Regional Scale
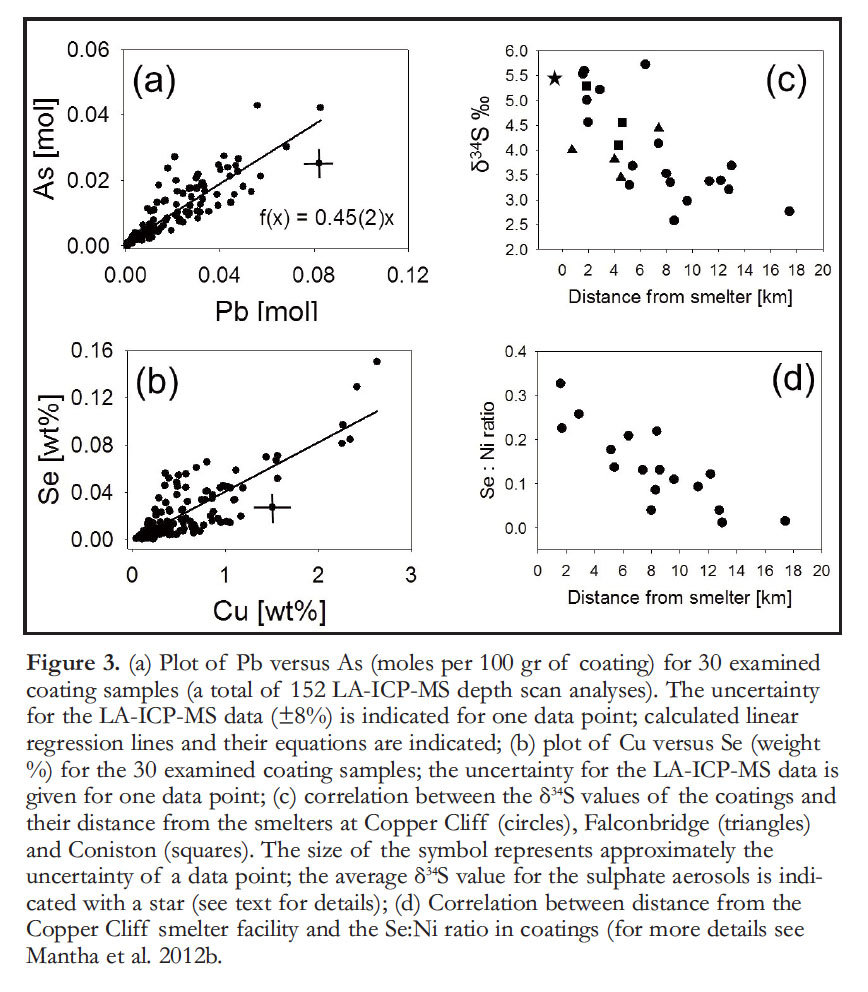
14 The concentrations of smelter-emitted metal(loid)s (Cu, Ni, Pb, As and Se) decrease in the coatings in proportion to the distance from the smelter point-sources (Mantha et al. 2012b), which agrees with results from previous studies on the distribution of metal(loid)s in soils (Freedman and Hutchinson 1980; Hogan and Wotton 1984; Wren 2012), peat (Zoltai 1988), lake sediments (Jackson 1978),snow(Telmer et al. 2004) and humus and till sediments (Henderson et al. 1998). However, correlations between concentrations of elements in coatings from different locations only occur if these elements are also closely associated in the coatings. For example, chemical distribution maps indicate the strong association of Pb–As and Cu–Se in the coatings and in the weathered portion of the underlying rock. Similarly, 152 LAICP-MS depth-scan analyses indicate a close association of Pb with As and Cu with Se in all examined coatings, independent of the geographic location or the type of underlying rock (Fig. 3a, b).
15 Copper is one of the major elements in the metal-sulphate-rich layer (Fig. 2d), whereas Ni is typically found within spherical Fe-oxide smelter particulate matter (Mantha et al. 2012a; Lanteigne et al. 2012; Fig. 2a). Consequently, the concentrations of Ni and Cu do not correlate in all coatings of the Greater Sudbury area (Mantha et al. 2012b), which can be attributed to variations in the proportions of spherical particulate matter and metal-sulphate-rich layers with the type of rock substrate and the geographic location of the coating (Mantha et al. 2012a).
16 These correlations as well as differences between ratios of elements in the coatings and smelter plume (based on published emission data; Wren 2012) indicate that the elemental ratios in the coatings were mainly controlled by the occurrence of minerals in the sulphate-rich layers and the ability of those minerals to incorporate trace metals based on their atomic size, coordination and charge (Mantha et al. 2012b).
 Display large image of Figure 3
Display large image of Figure 3Change in Isotope Composition on the Regional Scale
17 An important fingerprinting tool for identifying sources of pollutants once present in the atmosphere is the identification of isotopic compositions of anthropogenic SOx . Several studies have examined preserved traces of atmospheric pollutants in urban areas (e.g. Norman et al. 2006; Sinha et al. 2008), and around coal-fired power plants (e.g. Forrest and Newman 1977) and smelters (Nriagu and Coker 1978), as well as to interpret the related formation of weathering crusts on building stones (Buzek and Šrámek 1985).
18 The sulphur isotope composition (δ34S) of the coatings in the Greater Sudbury area varies between +2.6‰ and +5.7‰. The higher values are associated with samples collected in closer proximity to the smelting centres, notably Copper Cliff and Coniston. A correlation between the δ34S values of the coatings and their distance to the smelters was established through consideration of the prevailing southwest-to-northeast wind direction and emission data from the various smelters. Historical emission data from the smelters at Copper Cliff, Falcon-bridge and Coniston and the prevailing wind direction suggested that all coatings sampled west of Coniston and Falconbridge were predominantly affected by emissions from the Copper Cliff smelters, and that only coatings sampled around Coniston and Falcon-bridge were significantly affected by the emissions of the stacks in these areas. In this way, distances between sample locations and the Copper Cliff smelter center were calculated for three areas west of Coniston and Falcon-bridge (Sudbury Centre, Sudbury South, and Copper Cliff; Fig. 1a), whereas distances to the Coniston and Falconbridge stacks were only calculated for sampling locations in their vicinity. Figure 3c indicates a good correlation between the δ34S values of the coatings and their distances to the various smelters.
Coatings: Geological Records of Past Atmospheric Processes
19 The regional distribution of metal(loid)s and sulphur isotopes in black rock-coatings are the result of atmospheric processes such as wet and dry deposition, mixing of aerosols and scavenging effects. These processes and their preservation in the coatings reflect the speciation of metal(loid)s in the emission plume and the deposition and mixing of different sulphur-bearing atmospheric species. Information about the nature and fate of atmospheric sulphur species can be gained by comparing the δ34S values of the coatings with those of the smelter plume and precipitations, and by evaluating the possibility of sulphur fractionation during oxidation of atmospheric SO2.
Sulphur Isotope Composition of SO2and Sulphates in the Smelter Plume and Wet Precipitations
20 Smelters in the Greater Sudbury are known to release both SO2 gas and sulphate-bearing particulate matter (Forrest and Newman 1977; Wren 2012). Stable isotope studies of the main smelter plume (the Super Stack) documented that SO2 in the plume displayed low positive δ34S values of +0.9‰ to +1.2‰ (Nriagu and Coker 1978), which is consistent with the isotopic composition of most of the smelted Ni–Cu ores (+0.2‰ to +3.5‰) in this region (Thode et al. 1962; Schwarcz 1973). Sulphates in the smelter plume mainly occur in the form of H2SO4, plus minor amounts of metal-sulphate aerosols. The proportion of these primary sulphates in the plume varies between 0.4 to 3% of total SO2 emissions (Chan et al. 1982a, b; 1983) and their δ34S values are in the range of +4.2 to +7.6‰ (Lusis and Wiebe 1976).
21 Studies on the conversion of SO2 to H2SO4 indicate that the oxidation rate of SO2 is on average 1% per hour in the main smelter plume (Lusis and Wiebe 1976; Forrest and Newman 1977). These studies further indicate that the smelter plume travelled, on average, 40 km per hour. Sample locations of the black coatings were all within 40 km of the smelter centres, thus the isotopic signature of sulphates deposited in this area was significantly influenced by primary sulphates (+4.2 to +7.6‰) and their mixing with secondary sulphates (i.e. sulphates formed from the oxidation of atmospheric SO2).
22 Chan et al.(1982c,d,e) and Chan and Lusis (1985) further demonstrated that sulphates and trace metals from the plume are efficiently scavenged during wet precipitation events. For example, typical scavenging coefficients for sulphates were found to be about 30 to 40% per hour, and for trace metals such as Pb, Cu, Se and As, 60 to 80% per hour (see below). The same studies also suggest that the scavenging coefficient for SO2 was insignificantly small relative to that of sulphates, indicating that exposed rocks in the Sudbury area mainly interacted with primary and secondary sulphates scavenged via wet precipitation.
Did Fractionation of Sulphur Occur?
23 The fractionation of S isotopes in the atmosphere can be mediated by a gas-phase or an aqueous-phase oxidation process. The gas-phase oxidation (also known as homogeneous oxidation) occurs via OH radicals in the tropo-sphere and stratosphere and produces sulphuric acid (H2SO4), which condenses to form aqueous (SO4)2- and (SO4)2- -bearing aerosols (Saltzman et al. 1983; Tanaka et al. 1994). The aqueous-phase oxidation (also known as heterogeneous oxidation) occurs in the aqueous component or on particle surfaces in the atmosphere. The major oxidants are H2O2, O3 and O2, the latter being catalyzed by Fe3+ and other transition metal ions (Herrmann et al. 2000).
24 The isotopic composition of secondary sulphates (δ34SH2SO4 ) formed during Rayleigh distillation can be cal culated using the following equation:
25 δ34SH2SO4 = α (δ34S 0SO2 + 103)fα-1 - 103 where δ34S0SO2 is the initial δ34S value for SO2 (average value of SO2 in the plume = 1.11‰ for f = 1), f the fraction of SO2 remaining (Faure 1998), and α is the fractionation factor for a gas-phase or aqueous-phase oxidation process (for details see Mantha et al. 2012b).
26 Using the above equation and reported fractionation factors for gas-phase or aqueous-phase oxidation processes, Mantha et al. (2012b) showed that secondary sulphates would have either large positive (>11‰) or negative (<-9‰) δ34S values if the remaining fraction of SO2 decreases in steps of 0.01 (corresponding to an oxidation rate of 1% per hour). Hence, mixing of similar proportions of primary sulphates (+4.2 to +7.6‰) with secondary sulphates containing fractionated sulphur (values of either >11‰ or <-9‰) would result in 34SH2SO4 values that are not consistent with those measured in the coatings (+2.6 to +5.7‰). As a result, sulphur fractionation during gas-phase or aqueous-phase oxidation processes was disregarded as the cause of the decrease of δ34S with distance from the smelters.
Mixing of Primary and Secondary Sulphates
27 The content of primary sulphates in the emission plume(s) ranges between 0.4 to 3.0% (Chan et al. 1982c, d, e). Hence, an SO2 oxidation rate of 1% per hour would result in approximately equal amounts of primary and secondary sulphates within the first two hours after emission of the plume. The average δ34S for primary sulphates (5.4‰) is similar to the δ34S values observed in the coatings proximal to the smelters (Fig. 3c). If gas-phase or aqueous-phase oxidation processes did not result in fractionation of sulphur isotopes, as observed in the urban areas of Calgary and Vancouver (Norman et al. 2004a, b), the δ34S for the secondary sulphates would be similar to those for the SO2 (average value = 1.11‰) from which they formed. Two processes occurred now simultaneously in the smelter plume with increasing distance to the smelter: a) Primary sulphates were scavengers by wet precipitation resulting in a decrease of sulphates with δ34S = 5.4‰; and b) Oxidation of SO2 resulted in an increase of secondary sulphates with δ34S = 1.11‰. These two processes yielded to an increasing ratio of secondary (δ34S = 1.11‰) to primary sulphates (δ34S = 5.4‰) in the smelter plume and thus in a decrease in the δ34S of atmospheric sulphates (and consequently of sulphur species in the coatings) with increasing distance from the smelters. This mixing model was verified through inspection of the regional distribution of trace metals in the coatings, which were found to be closely associated with primary sulphate aerosols in the smelter plume.
Deposition Rates of Sulphate Aerosols and Larger Particulate Matter
28 Studies of particulate matter in the Copper Cliff smelter plume found that Pb, As and Se occurred predominantly in small sulphate aerosols having average diameters <2.5 µm, compared to Cu and Ni, which were found mainly in larger particulate matter (>2.5 µm; Chan et al. 1982a, b). Studies on the wet and dry deposition rates of metals from a smelter plume indicate that deposition rates for the larger Fe–Ni particulate matter are similar during wet and dry conditions, whereas the rates for the smaller metal-sulphate aerosols dramatically increase during wet deposition (Chan et al. 1982c,d,e). These results indicate that during precipitation events, the travelling smelter plumes became depleted in Pb-, Asand Se-aerosols with increasing distance to the stacks. Hence, precipitation and consequently coatings closer to the smelters became more enriched in Pb-, As- and Se-bearing metal-sulphates relative to Fe–Ni-bearing particulate matter, than precipitation and coatings farther away from the smelters. Plots of Pb/Ni, As/Ni, and Se/Ni ratios in coatings versus their distance to the Copper Cliff smelter indicate that coatings closer to the smelter are indeed enriched in Pb, As and Se relative to Ni (Mantha et al. 2012b; Fig. 3d). Furthermore, the ratio of primary Pb–As–Se-bearing sulphates to secondary sulphates decreases with distance from the smelter, which is in accordance with the interpretation of the decrease in δ34S with distance from the point source.
CONCLUSIONS AND OUTLOOK
29 Our studies on the black coatings of the Greater Sudbury area indicate that silica coatings are remarkable geological records of nanoparticles emitted to the atmosphere as well as atmospheric processes such as mixing, deposition and scavenging. Detailed mineralogical and chemical characterization of the coatings indicated the following characteristic features of silica coatings in their gelatinous state:
i) they contain a connected interstitial pore system that allowed the in situ formation of secondary minerals and the mixing of pore fluids;
ii) they have a low viscosity that allowed the trapping and entombment of nano- to micro-size particulate matter; and
iii) their nanometre-size pores and adsorbed silica species allowed the encapsulation and preservation of nanoparticles and soluble acidic and basic Fe–Cu-sulphates.
30 On a regional scale, the coatings display a decrease in metal concentrations, an increase in the ratio of secondary to primary sulphates, and a decrease in the δ34S of primary and secondary atmospheric sulphates with increasing distance from the smelter point-sources. The coatings also record:
i) higher scavenging and thus higher deposition rates of primary sulphates containing Pb, As and Se by wet precipitations; and
ii) similar deposition rates of larger Ni-bearing particulate matter under both wet and dry conditions.
A comparison of emission data and elemental ratios between Pb, As, Cu and Ni ratios in the coatings showed that the ratios are mainly controlled by the occurrence of minerals in the sulphate-rich layers and their ability to incorporate trace metals according to their atomic size, coordination and charge (Mantha et al. 2012b).
31 Another interesting aspect is that silica-rich coatings on Mars, which are believed to be products of the weathering of the underlying rock, contain sulphates and amorphous silica as their main constituents (Tosca et al. 2005; Tosca and McLennan 2006; Hurowitz et al. 2006). The present study has shown that silica and Fe-sulphate coatings preserve the isotopic signature of sulphur involved in their formation as well as differences between wet deposition rates for atmospheric sulphate species and particular matter. Similarly, silica coatings on Mars may contain information with respect to atmospheric sources and processes.
ACKNOWLEDGEMENTS
32 This work was supported by a NSERC Discovery grant to MS, and an Ontario Graduate Scholarship to NM. KK acknowledges financial support from NSERC, CFI and OCE. We would like to thank K. Klassen for her help at the Queen’s University Stable Isotope Lab. MH acknowledges NSF and EPA under NSF Cooperative Agreement EF-0830093. Any opinions, findings, conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF or the EPA. This work has not been subjected to EPA review and no official endorsement should be inferred. We would like to thank technical staff members at Laurentian University, Ontario GEOLABS, Queen’s University Stable Isotope Lab and at the Nanoscale Characterization and Fabrication Laboratory, ICTAS, Virginia Tech for their assistance. We also want to thank Editor Brendan Murphy and an anonymous reviewer for their comments.