Paul F. Hoffman Series
A Temperature-Dependent Positive Feedback on the Magnitude of Carbon Isotope Excursions
SUMMARY
The decrease in the average magnitude of carbon isotope excursions in marine carbonates over Phanerozoic time is a longstanding unresolved problem. In addition, carbon isotope excursions commonly co-occur with oxygen isotope excursions of the same sign, implying the existence of a longstanding link between organic carbon burial fluxes and temperature. It was proposed that this connection was provided by the thermodynamic relationship between temperature and microbial respiration rates – changes in temperature drive changes in organic carbon remineralization rate and organic carbon burial efficiency. Such a mechanism provides the logic for a positive feedback affecting the magnitude of both climate changes and carbon isotope excursions. Here, we employ feedback analysis to quantify the strength of this mechanism with modifications to a simple carbon isotope mass balance framework. We demonstrate that the potential strength of this feedback is large (perhaps several permil) for plausible ranges of historical climate change. Furthermore, our results highlight the importance of the surface temperature boundary condition on the magnitude of the expected carbon isotope excursion. Comparisons of our model predictions with data from the terminal Eocene and Late Ordovician (Hirnantian) greenhouse–icehouse climate transitions suggest that these excursions might be substantially explained by such a thermodynamic microbial respiration feedback. Consequently, we hypothesize that the observed pattern of decreasing excursion magnitude toward the present might be explained at least, in part, by a decrease in the mean temperature of environments of organic carbon burial driven by long-term climate and paleogeographic trends.
SOMMAIRE
La diminution de l'amplitude moyenne des excursions des isotopes du carbone dans les carbonates marins au fil du Phanérozoïque est une énigme de longue date. On note en outre que les excursions des isotopes du carbone coexistent couramment avec des excursions isotopiques de même signe de l'oxygène, ce qui implique l'existence d'un lien de longue date entre les flux d’enfouissement du carbone organique et la température. On a suggéré que ce lien découlait de la relation thermodynamique entre la température et les taux de respiration microbienne - les changements de température déterminent le taux de reminéralisation du carbone organique et l’efficacité de l’enfouissement du carbone organique. Un tel mécanisme peut expliquer la rétroaction positive affectant à la fois l'ampleur des changements climatiques et les excursions des isotopes du carbone. Dans le cas présent, nous utilisons l'analyse de la rétroaction pour quantifier la robustesse de ce mécanisme avec des modifications d’un simple bilan de masse des isotopes du carbone. Nous démontrons que la robustesse potentielle de cette rétroaction est forte (peut-être plusieurs pour mille) dans les gammes plausibles du changement climatique historique. De plus, nos résultats mettent en évidence l'importance de la condition aux limites de la température de surface sur l'ampleur de l'excursion isotopique du carbone attendue. Les comparaisons des prédictions de notre modèle avec les données de la fin de l'Éocène et de la fin de l’Ordovicien (Hirnantien) des transitions climatiques à effet de serreeffet/de glaciation permettent de penser que ces excursions pourraient être correctement expliquées par une telle rétroaction de la thermodynamique de la respiration microbienne. Par conséquent, nous émettons l'hypothèse que la tendance observée de diminution de l'ampleur de l’amplitude des excursions du passé vers le présent peut s'expliquer, au moins en partie, par une diminution de la température moyenne du milieu d'enfouissement du carbone organique engendrée par des tendances climatiques et paléogéographiques à long terme.
INTRODUCTION
1 Geologically rapid excursions in the carbon isotopic composition of marine carbonates and organic matter (δ13Ccarb, δ13Corg , respectively) are a conspicuous but still incompletely understood feature of the stratigraphic record. The δ13C trends record clear changes in the behaviour of the carbon cycle, but may reflect a variety of processes including volcanic activity, rock weathering, primary production, and organic carbon preservation; and many excursions likely record complex interactions between carbon cycle processes (Kump and Arthur 1999). Nevertheless, some commonalities can be observed that can help identify important, longstanding feedbacks.
2 Noting that Phanerozoic δ13C excursions commonly exhibit a positive correlation with δ18O excursions, Stanley (2010) suggested that many δ13C excursions were caused by climate change via the thermodynamic linkage between ambient temperature and the metabolic rates of microbial decomposers, using similar logic to biogeo-chemical models of marine production export efficiency (Laws et al. 2000). The idea is simple and useful: microbial respiration rates are strongly temperature-dependent, and so changes in ocean temperatures affect the efficiency of organic carbon export and burial, and can thereby drive changes in δ13C values. In this framework, climate cooling causes more efficient organic carbon burial and positive δ13C excursions; climate warming has the opposite effect and would cause negative excursions (Stanley 2010). As noted by Stanley (2010), this mechanism additionally provides a potential explanation for the commonly observed coincidence of δ13C excursions with extinction events in the fossil record.
3 One important aspect of Phanerozoic δ13C excursions that Stanley (2010) was unable to fully explain is the variation in their magnitudes over Earth history. He noted that, “It is puzzling, for example, that the terminal Eocene excursions were small despite having been associated with substantial global cooling.” In fact, the terminal Eocene excursion is one of the largest of the Cenozoic interval, but it is comparatively small in the context of Earth history. Indeed, one of the most striking features of the Phanerozoic carbon isotope record is a decline in the average magnitude of isotope ratio excursions through time (Grotzinger et al. 2011; Fig. 1). Here, we elaborate on Stanley’s (2010) hypothesis and draw attention to a possible explanation for the decrease in the magnitude of δ13C excursions observed over Phanerozoic time.
4 We hypothesize that a key aspect of the temperature–organic carbon burial feedback is its sensitivity to the initial boundary condition: due to the exponential nature of the relationship between environmental temperature and metabolic rates (Fig. 2A), the expected magnitude of change in organic carbon burial (and δ13C) depends not just on the sign and magnitude of temperature change but also, critically, on the baseline temperature from which temperature change initiates (hereafter referred to as initial temperature). It is possible to modify and employ simple isotope mass-balance calculations to estimate the potential effect strength (i.e. system gain) that this feedback might provide, and then reflect on these estimates by comparison to paleoclimate proxy data generated from the fossil record. The relative magnitudes of the terminal Eocene and Late Ordovician carbon isotope excursions – two events that record greenhouse–icehouse transitions – can be explained, in part, by proxy-based reconstructions of initial temperature and change in temperature during these events. Building on this result, we develop the hypothesis that one possible explanation for the decreasing magnitude of carbon isotope excursions throughout Phanerozoic time is the lower temperatures of organic carbon burial environments due to the overall cooling trend observed in proxies of seawater temperature, and to net movement of continents from tropical to temperate latitudes over this interval.
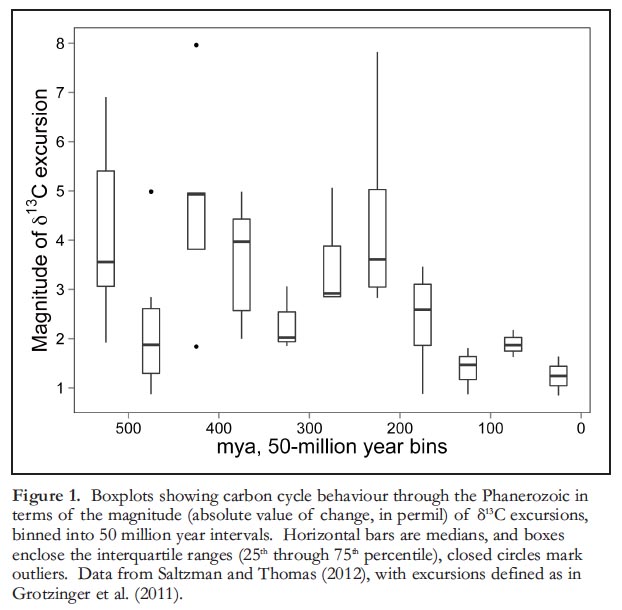 Display large image of Figure 1
Display large image of Figure 1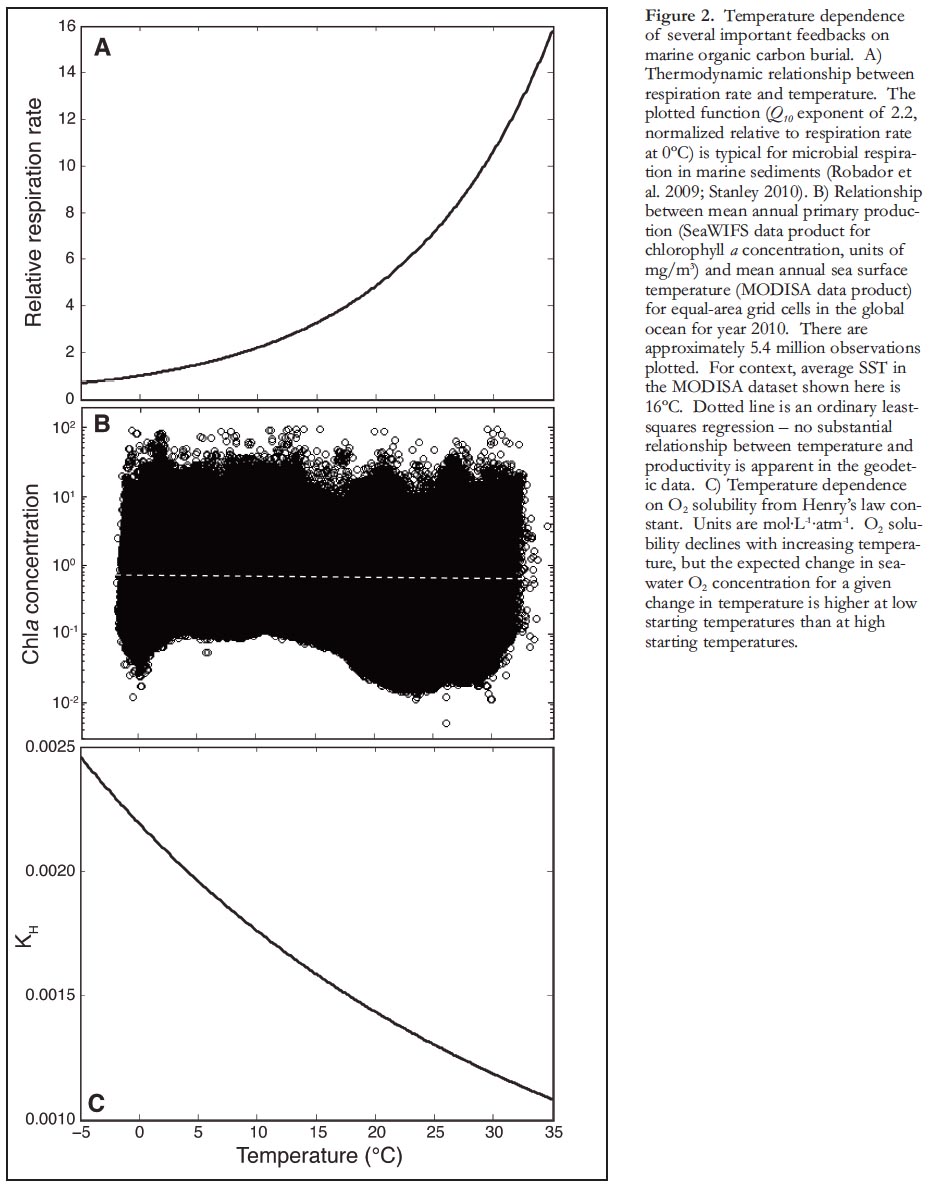 Display large image of Figure 2
Display large image of Figure 2FEEDBACK ANALYSIS AND EFFECT STRENGTH
5 It is useful to conceptualize the temperature-dependent organic carbon burial mechanism proposed by Stanley (2010) as a feedback in a dynamic system, rather than an external forcing mechanism, because many other factors can drive and influence δ13C trends in addition to climate (Jones and Jenkyns 2001; Svensen et al. 2004; Tejada et al. 2009). Some δ13C excursions may record changes in total carbon burial flux rather than merely changes in the ratio of organic to inorganic carbon burial (Brenchley et al. 1994; Saltzman and Young 2005), and in these cases climate changes may be a consequence, rather than a cause, of carbon cycle perturbation. Nonetheless, as long as a given set of changes in the fluid Earth carbon cycle affects temperature, even as a corollary of other drivers, a temperature-dependent thermodynamic feedback on organic carbon burial can be expected.
6 This thermodynamic feedback is positive, meaning it will tend to amplify the system response in both atmospheric pCO2 (and consequently climate) and in δ13C of marine carbonates. For example, it has been hypothesized that increased volcanic CO2 out-gassing at the Permian–Triassic boundary due to eruption of the Siberian Traps flood basalts produced a negative C isotope excursion (Payne and Kump 2007). In addition, however, warming associated with this CO2 injection – supported by proxy observations in Early Triassic paleoenvironments (Retallack 1999; Joachimski et al. 2012) – would have increased microbial respiration rates and decreased organic carbon burial, leading to a larger negative δ13C excursion (and climate perturbation) than expected from the carbon budget of the volcanic out-gassing alone. Similarly, the magnitudes of positive δ13C excursions attributed to external factors that would have also caused climate cooling (e.g. nutrient-driven organic carbon burial events (Saltzman 2005)) would have been amplified by the temperature-dependence of respiration.
7 The increase in respiration rates with temperature is clear in sign, but it is important to consider the effect strength. A useful approach to evaluate the effect of a particular feedback is to consider a simple reference system, and then estimate the system gain as the amount of amplification of the response due to the inclusion of feedback (Roe 2009). The simplest description of the geological carbon cycle is conveyed by classic steady-state isotope mass balance formulations that describe the δ13C values of carbonate and organic carbon as a function of the fraction of total carbon buried as organic carbon over a given interval of time (Summons and Hayes 1992; Kump and Arthur 1999). Formally, this system is not temperature sensitive until the thermodynamic feedback of Stanley (2010) is included. Stanley (2010) appreciated that the bigger the temperature change, the larger the effect on the carbon cycle (due to exponential differences in remineralization rates), but the nonlinearity between temperature and respiration rate means there is another important additional element that bears on the gain – the initial temperature boundary condition. This feedback is expected to be non-linear. Because global sea-water temperatures have almost certainly varied significantly over Phanerozoic time, it is important to estimate the impact this feedback might have had on the global carbon cycle as a function of both amount of temperature change and initial temperature.
8 We begin with a simple steady-state isotope mass balance framework and assume that the long-term, steady-state remineralization rate of organic carbon (Fremin) is tightly controlled by external factors [over long timescales (>106yr) the amount of remineralization is closely tied to production because of negative feedbacks in O2 and nutrient cycles (Lasaga and Ohmoto 2002)] and is independent of temperature. This assumption is supported by the observation that baseline values of δ13Ccarb are essentially invariant over Phanerozoic time (Saltzman and Thomas 2012) despite well-documented changes to background global climate and surface temperatures over this interval (Frakes et al. 1992). In contrast, short-term (~105-106yr) temperature changes will impact marine respiration rates (and δ13C) because of the observed thermodynamic (e.g. – 'Q10') dependence on the microbial metabolisms responsible for organic carbon remineralization (Robador et al. 2009; Stanley 2010). The exponent (i.e. strength) of this relationship varies among environments, but a typical open-marine value is ~2.2 (Fig. 2A; Robador et al. 2009; Stanley 2010). Thus, the change in organic carbon remineralization flux (ΔFremin) is expected to approximately double for a 10°C increase in temperature. It is also important to note that the absolute differences in respiration rates due to a change in temperature depend strongly on initial temperature (Fig. 2A).
9 In principle, and at organismal scales, the same thermodynamic effects that cause heterotrophs to respire faster at higher temperature also apply to primary producers. That is, holding all other factors constant, a given change in temperature should influence the metabolic rates and population growth rates of both heterotrophs and autotrophs (Gillooly et al. 2001; Savage et al. 2004). If both productivity and microbial respiration had similar positive exponential relationships to ambient temperature, there would be no net effect on fractional organic carbon burial. However, over even short timescales productivity is limited by light and nutrients (and grazing, phages, etc.). To quantify the relationship between temperature and productivity in the modern ocean we acquired geodetic data products for the year 2010 from the MODIS Aqua (sea surface temperature) and SeaWIFS (sea surface chlorophyll a concentration) missions, and coupled them to the same grid matrix using the software SeaDAS (http://seadas.gsfc.nasa.gov/) so they could be readily compared. The global observations show no significant relationship between primary production (as measured by chla concentration) and sea surface temperature (Fig. 2B) – if anything, the data support a slight negative relationship as more primary productivity is tied to upwelling of cold, nutrient-rich waters (chla = −0.0218T + 0.7964). Overall, while temperature changes will strongly impact rates of remineralization, primary productivity is expected to operate largely independent of temperature (or may even decline at higher temperatures due to reduced upwelling, e.g. Behrenfeld et al. 2006). Consequently, there is no strong relationship between mean surface temperature and mean annual productivity (Fprod ) in the modern ocean (Fig. 2B), and no consensus on the expected relationship, if any, between changes in climate and changes in primary productivity (Taucher and Oschlies 2011).
10 If we assume that Fprod does not vary as a strong function of temperature, it follows that the proportion of gross primary production that is remineralized (frem ) is expected to increase with temperature. The magnitude of the expected change in frem for a given change in temperature can be estimated by:
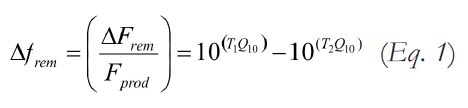
Where T1 represents the initial mean temperature, T2 the mean temperature following a climate change, and Q10 represents the mean relative change in global microbial respiration rate for a 10°C change in temperature. Here, we assume that remineralization scales as a simple function of global mean temperature, though the global remineralization flux may depend not just on the mean but also on the shape of the probability distribution function of global temperatures. Under a steady-state model with invariant Fprod , a change in relative remineralization rate must be offset by an equal but opposite change in organic carbon burial:
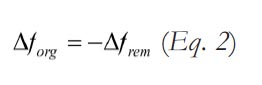
The corresponding change in the relative organic carbon burial flux (forg) will shift the stable isotopic composition of the marine dissolved inorganic carbon reservoir according to the following expression:
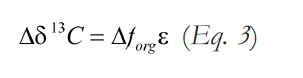
Where ε represents the fractionation factor (due to biological kinetic isotope effects) between coeval dissolved inorganic carbon and organic carbon in the oceans. The isotopic composition of marine DIC, in turn, is recorded in marine limestones as δ13Ccarb. Thus, using the above formulation, it is possible to relate the sign and magnitude of an isotope excursion in δ13Ccarb to a temperature-driven change in the efficiency of organic carbon respiration.
11 Critically, because the relationship between Frem and temperature is exponential rather than linear, the magnitude of δ13Ccarb excursion expected for a given change in mean temperature will also change as a function of initial mean temperature (T1). For example, cooling from a mean temperature of 25° to 20°C would be expected to generate a larger positive δ13Ccarb excursion than cooling from 20° to 15°C, which in turn would be expected to generate a larger positive δ13Ccarb excursion than cooling from 15° to 10°C.
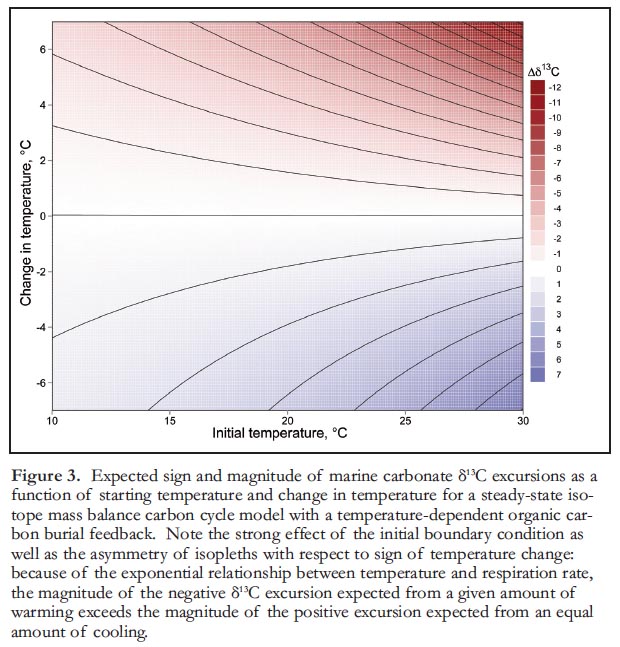
12 Figure 3 quantifies the nonlinear system gain from including a thermodynamic respiration rate-organic carbon burial feedback, in a classic geological carbon cycle isotope mass balance framework. These relationships illustrate the sign and magnitude of δ13Ccarb excursion expected for different combinations of initial temperature and change in temperature, assuming the same value for ε, here set at the Phanerozoic average of –29‰ (Hayes et al. 1999). Note that the available feedback strength estimates are potentially large (several ‰) for plausible ranges of historical Earth system climate changes. An additional interesting consequence of the exponential relationship between temperature and respiration rate is that the magnitude of negative δ13C excursion, expected from a given amount of warming, exceeds the magnitude of the positive excursion expected from an equal amount of cooling.
DISCUSSION AND CONCLUSIONS
13 There are many aspects of physical, chemical, and biological oceanographic systems that exhibit temperature dependence – some of these may drive positive or negative feedbacks on organic carbon remineralization rate that would act to amplify or damp the system response in addition to the feedback discussed above. One such feedback that is immediately apparent is the inverse relationship between O2 solubility (and concentration in seawater) and water temperature (Emerson and Hedges 2008; Fig. 2C). Because organic carbon preservation is strongly affected by oxygen availability (Hart-nett et al. 1998; Hedges et al. 1999), increased O2 concentrations at lower seawater temperatures could act as a negative feedback to counter the expected decrease in remineralization rate due to reduced microbial metabolic activity. Although the potential importance of this negative feedback is difficult to evaluate rigorously, we point out that the inverse relationship between O2 solubility and water temperature also has an exponential form (Fig. 2C) and will be differentially sensitive to an initial temperature boundary condition (i.e. dO2/dT is not a constant). Whereas relative changes in microbial carbon remineralization for a given change in temperature are expected to increase at higher baseline temperatures, relative changes in O2 solubility decrease at higher baseline temperatures. Thus, to the extent that a change in δ13CDIC reflects the net effects of changes in microbial metabolic activity and changes in oxygen availability on Frem, the magnitude of these changes is still expected to depend strongly on initial temperature.
 Display large image of Figure 3
Display large image of Figure 314 Temperature changes could also influence the delivery of O2 to bottom waters and sediments via changes in ocean circulation, but for a given change in temperature it is, at present, difficult to predict even the sign, much less the magnitude, of expected changes in the ocean mixing processes (IPCC 2007). Furthermore, changes in the mean temperature of the global oceans would not be felt equally everywhere – the magnitude of 13CDIC change expected would likely depend disproportionately on coastal temperature changes, and changes in seasonality in the high-productivity regions where most organic carbon burial and remineralization takes place. Because most of organic carbon burial takes place on continental shelves (Reimers et al. 1992), especially in deltaic systems (McKee et al. 2004), and organic carbon remineralization is concentrated in the upper 1 km of the ocean (Jørgensen and Kasten 2006), δ13CDIC changes will be especially sensitive to temperature changes in shallow ocean basins.
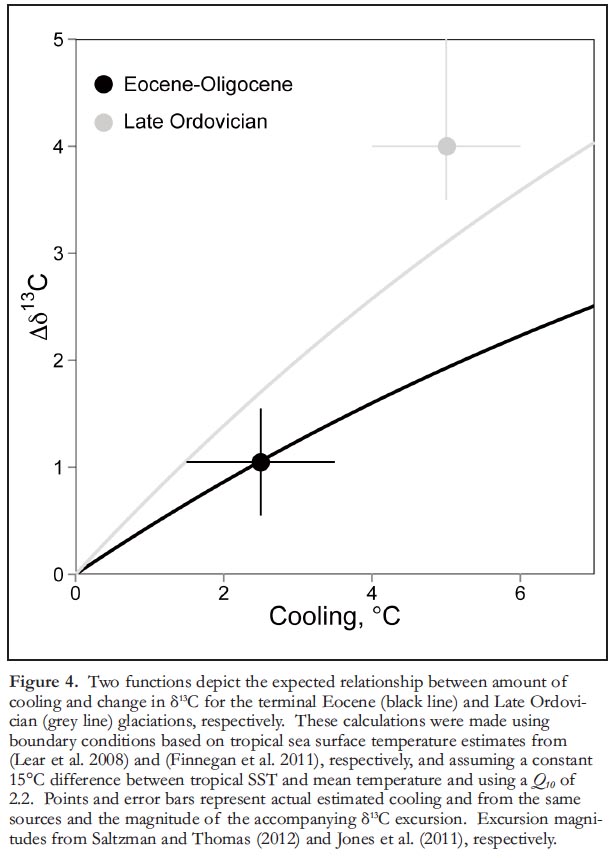
15 Though the calculations explored above do not represent a comprehensive biogeochemical view of the geological carbon cycle, the approach can be used to predict the sign and magnitude of the δ13Ccarb excursion accompanying a rapid change in mean ocean temperature, if T1, T2, Q10, and ε are known. And the expectations can be compared to geological observations of two well-known carbon isotopic excursions in the Phanerozoic record—both of which are known to be associated with transitions from relatively warm conditions to glacial ’icehouse’ states but are separated by more than 400 million years: the Paleogene Eocene–Oligocene boundary excursion and the Late Ordovician Hirnantian excursion.
16 As noted above, Stanley (2010) singled out the Eocene–Oligocene event as puzzling because the climate cooling produced only a relatively small ~1‰ positive δ13Ccarb excursion. The Mg/Ca data from planktic foraminifera suggest that tropical surface waters cooled by about 2.5°C across this boundary, from ~30.5° to 28°C. Although we have some constraints on tropic SSTs from proxy data, we do not know the distribution of temperatures in the upper kilometre of the oceans with any certainty. Consequently, we make the simplifying assumption (based on the distribution of temperatures in the 2010 MODISA dataset) that the difference between the mean temperature of tropical surface waters and the mean temperature of the upper 1 km of the ocean was the same in the Eocene–Oligocene as it is today – about 15°C (Robador et al. 2009; Locarnini et al. 2010). Using a typical early Cenozoic ε value of 29‰ (Hayes et al. 1999), a mean Q10 exponent of 2.2, and assuming a change in mean temperature from 15.5° to 13°C, our model predicts a positive δ13Ccarb excursion of nearly 1‰ (Fig. 4). For the Hirnantian glacial maximum, we make the same calculation with a somewhat higher initial mean temperature for the upper oceans of 20°C. This assumes the same 15°C difference between tropical surface temperatures and the mean temperature of the upper 1 km of the oceans as above, and a mean tropical sea surface temperature of 35°C based on clumped isotope measurements of well-preserved, shallow-marine carbonates (Finnegan et al. 2011). Using ε of 29‰ (a value typical for this interval (Hayes et al. 1999; Jones et al. 2011)), the observed 5°C cooling predicts an excursion of roughly 3‰ (Fig. 4). This estimate is slightly less than the observed excursion, which varies from 4–6‰ globally (Brenchley et al. 2003; Jones et al. 2011), but implies that reduced organic carbon remineralization driven by cooling can plausibly account for a substantial portion of this large positive excursion. This predicted value also substantially exceeds the ~1.7‰ excursion that would be predicted for a similar drop in temperature using the Late Eocene mean temperature estimate (Fig. 4).
 Display large image of Figure 4
Display large image of Figure 417 Although we only have sufficient data to rigorously consider two examples from opposite ends of the Phanerozoic Eon, a temperature-dependent organic carbon burial mechanism may help to explain the relative magnitudes of other conspicuous δ13C excursions observed in the Phanerozoic record. It is notable, for example, that some of the largest post-Cambrian carbon isotope excursions – both positive and negative –occur during the Early Triassic (Payne et al. 2004), an interval that appears to have been exceptionally warm (Retallack 1999). This feedback could also help to explain the overall decline in the average magnitude of δ13C excursions over Phanerozoic time, if the average temperature of organic carbon burial environments also declined over the same interval. Several lines of reasoning suggest this was the case.
18 The mean δ18O of benthic calcifiers has increased by more than several permil since Cambrian time (Prokoph et al. 2008), with similar trends observed in marine cherts (Karhu and Epstein 1986), and phosphates (Luz et al. 1984; Karhu and Epstein 1986; Trotter et al. 2008). This pattern implies either a substantial cooling of the seawater temperatures or a systematic increase in the δ18O of seawater through exchange with oceanic crust or some other mechanism (Jaffrés et al. 2007). Robust interpretation of this trend remains controversial (e.g. Giles 2012), but recent new analytical approaches using the clumped isotope proxy applied to Ordovician–Silurian carbonates (Came et al. 2007; Finnegan et al. 2011) reconstruct warm tropical ocean temperatures (e.g. 30° – 39°C) and do not support the hypothesis of exceptionally light early Paleozoic sea-water δ18O values. Although these clumped isotope studies provide broad support for the hypothesis of long-term Phanerozoic climate cooling (interposed by episodes of glaciation), coverage of the sedimentary record is currently limited and work is ongoing to better understand how this new proxy records climate information over geological time in a regime of ever-present carbonate diagenesis.
19 Another line of logic also suggests that a general decline in the average temperature of organic carbon burial environments occurred – even in the hypothetical case that average surface temperatures have been constant throughout the Phanerozoic. This is due to the changing distribution of continents through time and to secular changes in the nature and environments of organic carbon burial (e.g. Scotese 2001; Peters 2006). Most organic carbon burial takes place on continental margins, and consequently the meridional distribution of continental shelf and slope area captures a first-order control on global mean carbon remineralization rates. Given the general movement of continents from tropical to temperate latitudes following the Triassic breakup of Pangea (Scotese 2001), it is reasonable to conclude that most carbon burial today takes place under cooler environmental temperatures than it did during much of Paleozoic time.
20 We emphasize the utility of thinking about this temperature-dependent organic carbon burial mechanism as an internal feedback operating in the carbon cycle that may amplify the system response (as observed by the magnitude of δ13C excursions) to a given perturbation. What we find particularly surprising is that the strength of this feedback could be quite large. Our estimates of effect strength reveal that this feedback may play a first order role in carbon isotope excursions, and explain a substantial portion (up to several permil) of the magnitude of a given excursion. While these estimates may not remain as large when considered more comprehensively in more complex biogeochemical models that include other (positive and negative) feedbacks (e.g. Ridgwell and Zeebe 2005), we would expect the effect to remain important and ever-present. More specifically, the dependence of this thermodynamic organic carbon burial feedback on the initial temperature boundary condition raises the possibility that the decreasing magnitude of carbon isotope excursions over time may be due, at least in part, to long-term Phanerozoic climate and geodynamic trends.
21 Finally, reflecting on carbon isotope trends through all of Earth history, the volatile Neoproterozoic record (Knoll et al. 1986; Halverson et al. 2005) highlights a yawning divide between the ways in which Phanerozoic climate and the carbon cycle interacted and that of earlier Earth history. Although Proterozoic paleotemperature proxy estimates do not yet exist that allow direct comparisons to be drawn, it appears clear that the observed sign is opposite of expected – Neoproterozoic glaciations coincide with large negative (Hoffman et al. 1998; Rose et al. 2012), rather than positive carbon isotope excursions. To the degree that the temperature-dependent feedback discussed here is correct, it must have been overridden by stronger drivers during much of Neoproterozoic time (Schrag et al. 2002; Tziperman et al. 2011). Uncovering the nature of these differences will be critical to understanding the behaviour and evolution of climate and the carbon cycle over long intervals of Earth history.
We wish to express our gratitude to Paul Hoffman who introduced us to, and greatly stimulated our interests in, paleoclimate and the history of the carbon cycle during many late nights in Cambridge, MA. We thank Andy Thompson and Ian Eisenman for insight into the complexities of predicting ocean circulation changes as a function of temperature. The observations in Fig. 2B, collected and made available by the MODIS and SeaWIFS missions, were made possible, in part, by NASA. This work was supported by Agouron Institute awards to WWF and DAF, Packard Fellowships to WWF and DAF, and a National Science Foundation (EAR-1053523) award to WWF.