Series
Great Canadian Lagerstätten 2.
Macro- and Microfossils of the Mount Cap Formation (Early and Middle Cambrian, Northwest Territories)
SUMMARY
The Early–Middle Cambrian Mount Cap Formation, NWT, hosts a diverse range of exceptionally preserved fos sils. Like the celebrated Burgess Shale of British Columbia, the Mount Cap contains carbonaceous compression fossils of animals that lacked mineral ized hard parts, as well as the fully articulated skeletons of shelly taxa. Its unique importance, however, lies in exceptional carbonaceous preservation at a microscopic scale. Acid-extracted microfossils from the ‘Little Bear biota’ of the Mackenzie Mountains reveal important details of problematic groups including chancelloriids and hyolithids, and provide direct evidence of Cambrian diets in the form of fae cal strings. A complementary microfos sil assemblage from the subsurface of the Colville Hills region contains an extraordinary diversity of exquisitely preserved arthropod cuticle, and con stitutes the oldest known record of complex crown-group crustaceans. We discuss the wider significance of the Mount Cap fossils, and describe some new forms that point to the potential for future discoveries.
SOMMAIRE
La Formation de Mount Cap dans les T.N.-O. qui va du début de Cambrien jusqu’au Cambrien moyen renferme une gamme diverse de fossiles excep tionnellement bien préservés. Comme dans le cas des schistes de Burgess de Colombie-Britannique, la Formation de Mount Cap renferme des fossiles de compression carbonés d’animaux exempts de parties dures minéralisées, de même que de squelettes pleinement articulés de taxons coquillers. Cepen dant, son importance unique tient à sa préservation carbonée exceptionnelle à l’échelle microscopique. Les microfos siles obtenus par extraction à l’acide sur le « biote de Little Bear » des monts Mackenzie montrent d’impor tants détails sur des groupes controver sés incluant les chancelloriidés et les hyolithidés, ainsi que des indices directs de la diète cambrienne sous la forme de trainées fécales. Un assemblage microfossile complémentaire du sous sol de la région de Colville contient une extraordinaire diversité de cuticules d’arthropode très finement préservées, constituant ainsi la plus ancienne archive du groupe-couronne complexe de crustacés. Nous commentons à grands traits la signification de l’exis tence des fossiles de Mount Cap, et décrivons quelques formes nouvelles qui laissent penser que d’autres décou vertes sont possibles.
INTRODUCTION
1 The Cambrian ‘explosion’ of diverse shelly fossils and sedimentary traces marks the onset of the modern Phanerozoic biosphere, but presents a deeply unrepresentative account of the underlying diversity and dynamics. Many important evolutionary innovations were limited to ‘soft’ body parts and organisms, which only became fossilized in rare instances of ‘exceptional preservation’ – most famously in the Burgess Shale, which was discovered in SE British Columbia in 1909 (see Collins 2009). Since then, comparable and complementary fossil-Lagerstätten have been described from China, Greenland, Australia, the United States and elsewhere in Canada (see Hagadorn 2002). Although ‘Burgess Shale-type’ (BST) biotas are particularly well documented in the southern and central Canadian Rockies (e.g. Collins et al. 1983; Copeland 1993; Butterfield 2000; Johnston et al. 2009; Caron et al. 2010), they have also been encountered much farther north, in the Early to Middle Cambrian Mount Cap Formation of the Northwest Territories (Butterfield 1994; Butterfield and Nicholas 1996; Harvey and Butterfield 2008). As well as being of paleogeographic significance, the Mount Cap fossils have provided a unique view of early metazoan evolution via their (exceptional) preservation of micro-anatomy, and yield both key data points and an important new paleontological search image. Here we present a current summary of the Mount Cap biota, and champion its place among ‘great Canadian Lagerstätten.’
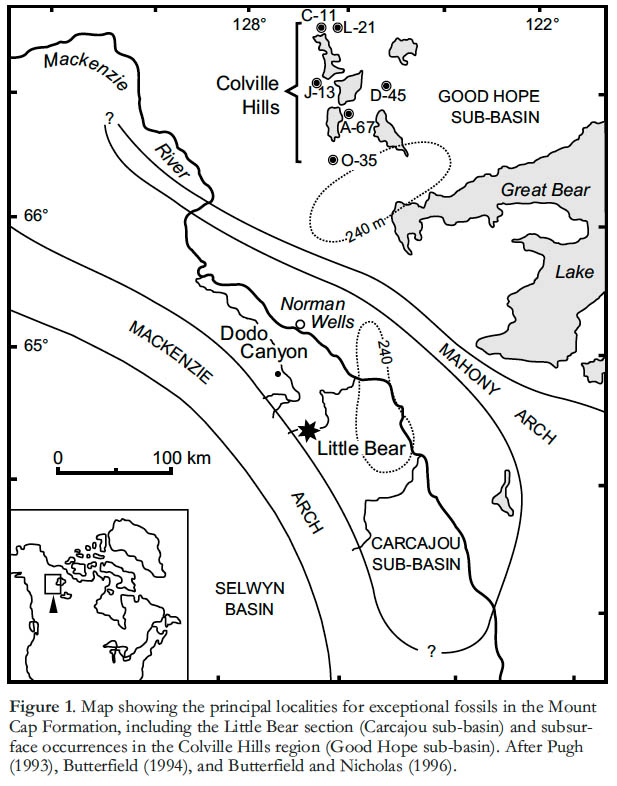
GEOLOGICAL SETTING
2 Unlike most of the units bearing BST assemblages to the south, the Mount Cap Formation was deposited in an intracratonic basin, separated from the open ocean to the west by the Mackenzie Arch (Fig. 1). The sedimentary succession is represented by 100–300 metres of mostly shallow water glauconitic sandstones, siltstones, shales and minor carbonates that crop out discontinuously in the Mackenzie and Franklin mountain belts (Aitken et al. 1973; Serié et al. 2009), and are intersected locally in the subsurface by exploration boreholes (Pugh 1993; Wielens et al. 1990; Dixon and Stasiuk 1998). The NW–SE trending Mahony Arch defines two localized depocentres: the Carcajou sub-basin, which includes the cordilleran outcrops; and the Good Hope sub-basin, which lies to the east of any orogenic deformation and is known primarily from drillcore (Fig. 1). In both areas, trilobite biostratigraphy identifies a late Early Cambrian (Bonnia-Olenellus Zone) to Middle Cambrian (Glossopleura Zone) depositional age for the Mount Cap Formation.
 Display large image of Figure 1
Display large image of Figure 13 Burgess Shale-type fossils were first discovered in drillcore from the Colville Hills area (Butterfield 1994) and only subsequently identified in outcrop, some 300 km to the south, in the front ranges of the Mackenzie Mountains (Butterfield and Nicholas 1996). Because of their marked geographical, geological and paleontological differences, we recognize two distinct fossil assemblages: the Early Cambrian ‘Colville Hills biota’ (Good Hope sub-basin), and the Middle Cambrian ‘Little Bear biota’ (Carcajou subbasin).
THE LITTLE BEAR BIOTA: ‘BURGESS SHALE-TYPE’ FOSSILS AT MACROAND MICROSCOPIC SCALES
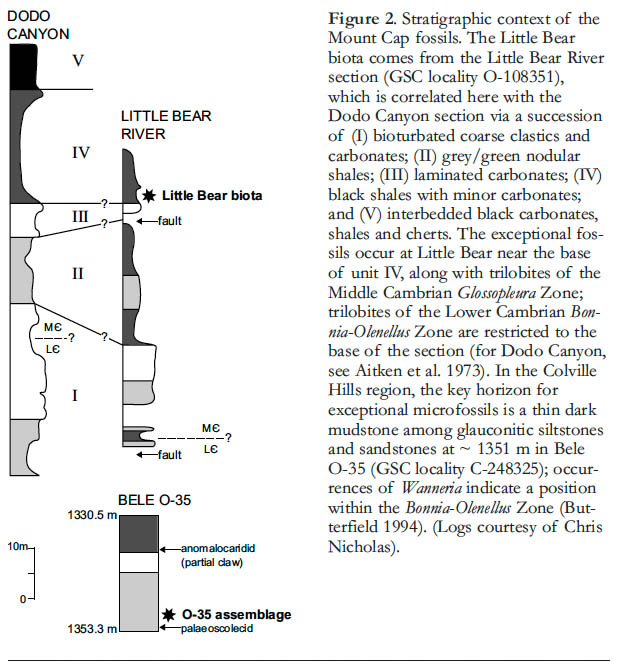
4 The Little Bear locality, on the Little Bear River in the Mackenzie Mountains (section U-13 of Aitken et al. 1973; section 2 of Serié et al. 2009; Fig. 2), has yielded a variety of exceptionally preserved fossils (Butterfield and Nicholas 1996). Entirely non-biomineralizing animals are represented by the claws of anomalocaridids (Fig. 3a) and the carapaces of bivalved arthropods, including Isoxys (Butterfield and Nicholas 1996, fig. 2.3). In addition, animals with mineralized skeletons that typically disarticulate after death are often preserved intact at Little Bear; examples include trilobites, sponges, and two problematic extinct groups: the mollusc-like hyolithids (Fig. 3b) and sponge-like chancelloriids (Fig. 3c). The chancelloriids are preserved as articulated scleritomes with traces of integument, while the hyolithids often retain their operculum and one or both arm-like helens; one hyolithid preserves a simple looped gut (see Butterfield 2003, fig. 2A).
 Display large image of Figure 2
Display large image of Figure 2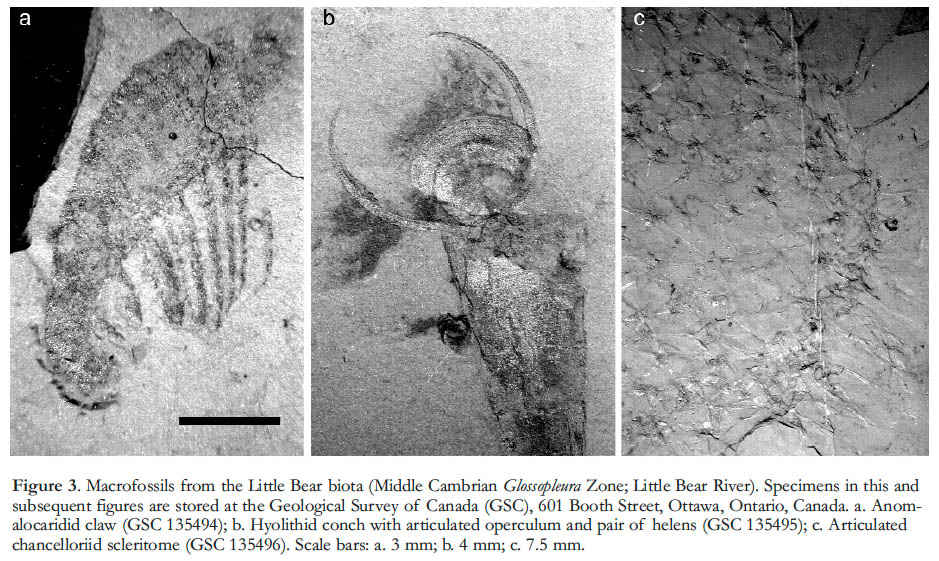 Display large image of Figure 3
Display large image of Figure 35 The Little Bear macrofossils are typically defined by a silvery carbonaceous film. Both the non-mineralizing and the shelly forms possess substantial films, indicating that even biomineralized body parts can follow a Burgess Shale-type taphonomic pathway under particular diagenetic conditions (Butterfield and Nicholas 1996). One consequence of this robust carbonaceous expression is that comparatively intact specimens can be isolated from the rock matrix using a gentle hydrofluoric acid technique. Such processing has revealed the fine-scale features of larger Little Bear fossils, and allows the recovery of small specimens that would otherwise go unnoticed.
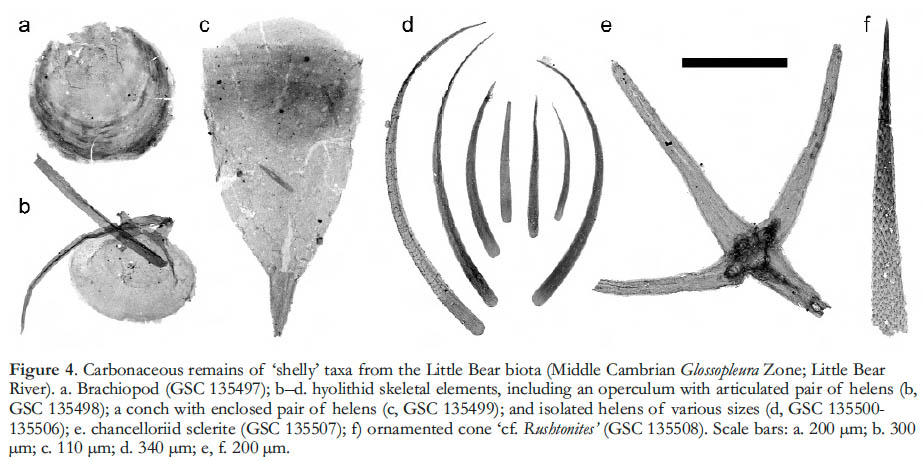
6 Significantly, the recovery of carbonaceous constituents in the Little Bear biota offers a complementary new view of familiar shelly fossils (Fig. 4). Cuticle fragments of olenellid trilobites, for example, reveal minute pores and the details of terrace ridges (Butterfield and Nicholas 1996, figs 3.1–2), while shell layers from organo-phosphatic brachiopods exhibit a concentric accretionary pattern and sometimes a porous or granular texture (Fig. 4a). More surprisingly, acid-extraction has recovered structures that had not been known to possess substantial carbonaceous components, including the isolated sclerites of chancelloriids (Fig. 4c) and the conchs, helens and opercula of hyolithids (Fig. 4b, d). In both cases, the carbonaceous specimens reveal details that are not evident in conventional shelly fossils, but bear on the mode of growth and, in turn, the phylogenetic affinities of these problematic groups (see Butterfield and Nicholas 1996; Butterfield 2003; Mus and Bergström 2007; Porter 2008; Harvey et al. in press). The assemblage also contains narrow ornamented cones that resemble the ‘small shelly fossils’ Rushtonites and Mongolitubulus (Fig. 4f). Like many of the smaller acid-extracted brachiopods, hyolithids and chancelloriid sclerites, the cones lack both remnant three-dimensionality and brittle fractures, and may have been only weakly mineralized in life, if at all. This implies a strong bias in the conventional (shelly) fossil records of these groups (Butterfield and Nicholas 1996).
7 In addition to body fossils, the Little Bear biota contains novel trace fossil evidence of diet and behaviour. Most notably, fecal pellets (and possibly other types of bromalite such as regurgitated material, or isolated gut contents) occur in a variety of sizes, shapes, and modes of preservation. At the macroscopic scale, Little Bear faecal pellets occur as either carbonaceous compressions (Butterfield and Nicholas 1996, fig. 2.4) or as three-dimensional phosphatized structures (Butterfield 2001, figs. 1.3.2.1e). The compression fossils exhibit regular ovoid outlines and a pseudo-segmented structure, but yield no direct clues to the identity of their producer or its diet. The phosphatized examples, in contrast, are elongate to round in outline and variably ‘segmented’ or spiriform; one specimen contains numerous brachiopod shell fragments and was clearly produced by a predator or scavenger.
 Display large image of Figure 4
Display large image of Figure 48 Fecal structures also occur among the acid-extracted microfossils (Butterfield and Nicholas 1996; Wilson 2004), where they take the form of more or less coherent strings of compressed carbonaceous material up to a few millimetres long and from 20 to 450 µm wide (n > 70). In overall shape they vary from straight to looped, coiled or sinuous (Fig. 5a–d), sometimes with an internal structure of aligned pellets (Fig. 5a). Most specimens consist of amorphous material of indeterminate origin, but a few are densely packed with sphaeromorphic acritarchs (Fig. 5d, e). The acritarchs are uniformly small (< 10 µm diameter), which suggests that they represent primary productivity (‘algal’ cysts) rather than (for example) the egg cases of metazoans, and so the pellets can be assigned to an herbivorous metazoan grazer, and possibly a member of the zooplankton. Although an alternative, benthic ecology is difficult to rule out, the high abundance of intact pellets in dark shale samples suggests that they were exported from an open water column overlying an undisturbed, probably anoxic sediment surface.
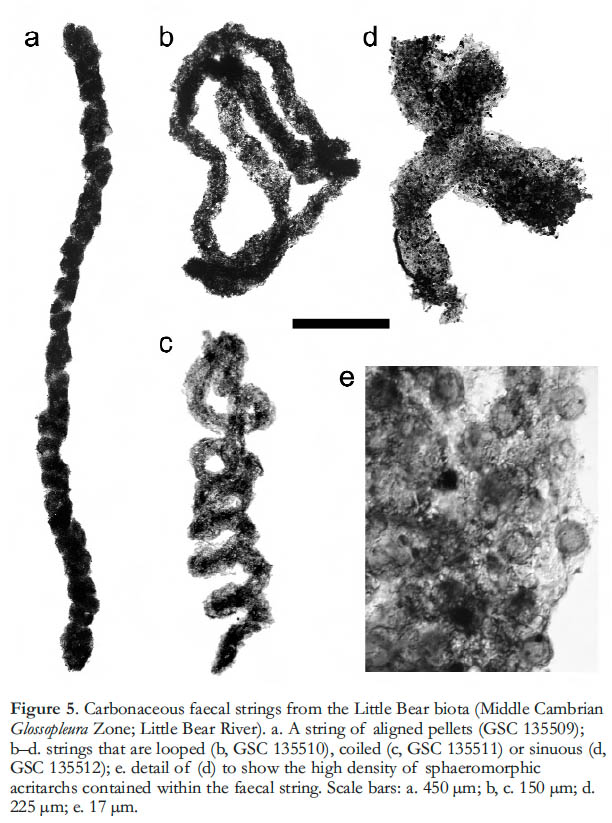 Display large image of Figure 5
Display large image of Figure 5THE COLVILLE HILLS BIOTA: SUBSURFACE MOUNT CAP LAGERSTÄTTEN
9 Of the seven boreholes that intersect the Mount Cap Formation in the Colville Hills area, we have identified Burgess Shale-type fossils in six, from numerous horizons (Butterfield 1994). Not surprisingly, macroscopic forms are rarely encountered in the cores, but include semi-articulated arrays of sponge spicules and, in Bele O-35, a poorly preserved anomalocaridid claw and a palaeoscolecid worm fragment (see Fig. 2). As in the Little Bear biota, however, it is the microfossils that stand out. Systematic acid processing has identified the widespread occurrence of Wiwaxia sclerites and Rushtonites-like spines in the subsurface, in rock types ranging from sandy, glauconitic siltstones to pale, medium and dark grey mudstones. By far the richest assemblage, however, comes from a single 5-cm-thick band of conspicuously dark and fine-grained mudstone in Bele O-35, at a depth of 1351 m (Fig. 2).This contains exceptionally well-preserved fragments of arthropod cuticle in extraordinary abundance, and arguably qualifies as a (micro) Lagerstätte in its own right.
10 Most of the O-35 arthropod fossils (Fig. 6a to r; n ~ 3800) consist of highly disarticulated appendage fragments. Even so, their original functions and anatomical positions can often be reconstructed by virtue of the exceptionally fine-scale (sub-micron) preservation of various cuticular specializations, including a great variety of spines, setae and setules (‘hairs’). Among the most informative specimens are groups of co-planar setae with a strict biserial arrangement of closely spaced setules (Fig. 6q), and various elongate, fringed elements (Fig. 6l–n) that bear a distinctive microstructure of aligned cuticular scales (Fig. 6r). Both structures have near-identical counterparts among living crustaceans, respectively in the filter plates used to collect suspended particles of food, and in the molar surfaces of mandibles (‘jaws’) that grind food prior to ingestion (Butterfield 1994; Harvey and Butterfield 2008).
 Display large image of Figure 6
Display large image of Figure 611 The particular style of molar ornamentation links the Mount Cap fossils to a clade of crown-group (pan)crustaceans that includes branchiopods, malacostracans, and hexapods, and thus provides a crucial calibration point for arthropod phylogeny, as well as the earliest record of an arthropod with a sophisticated particlefeeding ecology (Harvey and Butterfield 2008). Though now disarticulated, the molars and co-occurring filter plates and other armatures are likely to have constituted a single complex feeding apparatus in life. They would have worked together to generate, harvest and efficiently process extremely small particles of food – an impressive capability in an arthropod of relatively large body size (estimated length of 5 cm). Although the Mount Cap crustacean may have been active in the plankton (Butterfield 1994) – perhaps producing the sorts of acritarch-packed faecal strings that are known from the Little Bear biota – the various robust setal armatures suggest a lifestyle that involved benthic substrate-scraping activities, at least in part (Harvey and Butterfield 2008).
12 The exceptionally fine-scale preservation in the Mount Cap (O-35) assemblage has provided novel insights into the phylogeny and ecology of early crustaceans, even in the absence of articulated remains. This is partly because comparable detail is not preserved, or at least not observable, in the various macroscopic Cambrian fossils that have sometimes been interpreted as crustaceans, including a number of BST taxa (e.g. Hou and Bergström 1997; but see Walossek 1999; Budd 2008) and also a possible Cambrian phyllocarid preserved via coarse moulding in sandstone (Collette and Hagadorn 2010). The Mount Cap fossils also contrast with crustaceans known from phosphatic ‘Orsten-type’ preservation (e.g. Maas et al. 2006), which are similarly detailed but exhibit comparatively simple and unspecialized morphologies. This may be explained by the small body size of the Orsten individuals, which do not exceed 2 mm in length. In contrast, the Mount Cap fossils provide a microscopic view of macroscopic Cambrian crustaceans, and thus substantially augment our knowledge of early crustacean form, function, and phylogeny.
13 The O-35 assemblage is dominated overwhelmingly by crustacean remains, but has also yielded a modest diversity of unrelated fossils, several of which are attributable to lophotrochozoan animals (the group that includes annelids and molluscs, among others). The most abundant are sclerites of Wiwaxia (Fig. 7a; n = 111; Butterfield 1994), which are comparable in shape and microstructure to those from the Burgess Shale but are notably better preserved (cf. Butterfield 1990). The fine-scale structure of the sclerites, which is only apparent in acidisolated material, continues to figure prominently in discussions of lophotrochozoan phylogeny and the emergence of modern-type molluscs and annelids (Scheltema et al. 2003; Eibye-Jacobsen 2004; Butterfield 2006).
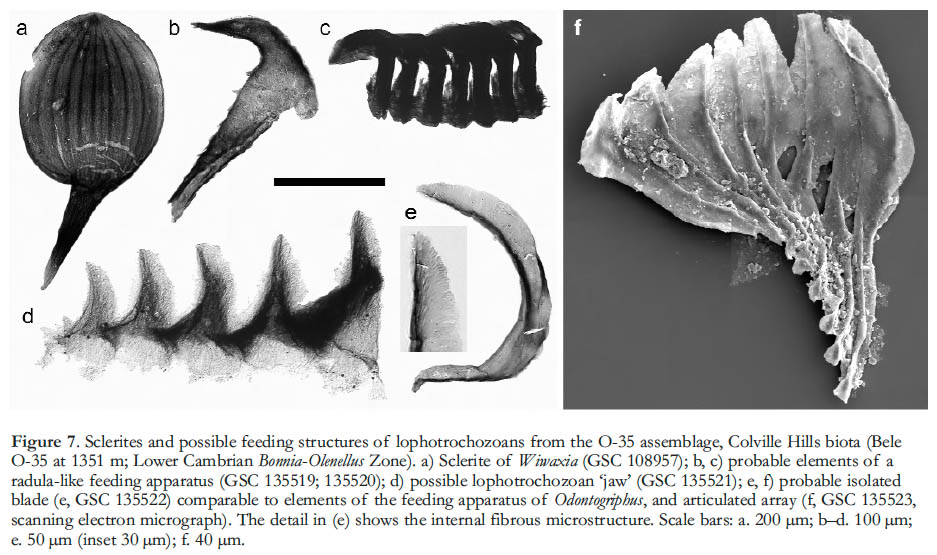 Display large image of Figure 7
Display large image of Figure 714 A small number of specimens in the Colville Hills biota apparently represent the feeding structures of early lophotrochozoans (Fig. 7b–f). Some can be assigned to a radula-like apparatus of a type known from the Early Cambrian Mahto Formation of Alberta (Butterfield 2008) and the Middle Cambrian Bright Angel Shale of Arizona (Strother et al. 2004). A single articulated array of seven ‘boot-shaped elements’ (Fig. 7c) is the most obviously comparable specimen (see Butterfield 2008, fig. 1), but there are also two recurved structures, one with secondary spination (Fig. 7b), that are similar to the ‘spinose elements’ of the Mahto assemblage (see Butterfield 2008, fig. 6). These specimens would be difficult to interpret without reference to the much larger Mahto sample, but can now be recognised as among the earliest radulas on record.
15 Two further specimens from the O-35 assemblage deserve attention as possible lophotrochozoan ‘jaws’. The first is at least superficially jaw-like in that it consists of a jagged array of five robust, conical size-graded ‘teeth’ (Fig. 7d). A lophotrochozoan affinity is hinted at by the construction of the teeth from apically directed fibres, which is suggestive of secretion by microvilli (see Butterfield 2008). By contrast, the second specimen is interpreted on the basis of its specific morphology as well as its general characteristics. It consists of a fan-shaped array of eight partially imbricating elements, each of which expands from a narrow base into a paddle- or leaf-shaped blade with a thickened longitudinal axis (Fig. 7f). Possible examples of similar but isolated blades from the O-35 sample (n = 3) have been viewed using transmitted light and reveal an underlying fibrous construction (Fig. 7e), consistent with lophotrochozoan-type secretion. Indeed, there are similarities in outline and microstructure to particular extracellular ‘tough parts’ in living polychaete annelids, including the fans of abdominal chaetae in various sabellids (although the Mount Cap elements are not fused proximally; cf. KnightJones and Fordy 1979, figs. 52–68), and the ‘maxillary plates’ of certain dorvilleids (ignoring the marginal serrations; cf. Tzetlin and Purschke 2005, fig. 10F, G). However, the closest comparisons are with the feeding apparatuses of Wiwaxia and Odontogriphus as they appear in articulated Burgess Shale macrofossils (see Butterfield 2006; Caron et al. 2006, 2007), which exhibit two (sometimes three) transverse ‘tooth rows’ of imbricating blade-like elements. The Mount Cap array conceivably represents one half of one such tooth row, based on its similar number of elements and their pattern of shape and length variation. Similarities in the finer scale characteristics of the ‘teeth’, in so far as they can be resolved in the Burgess Shale macrofossils, include the variably rounded or truncated shape of the broad end, and the thickened longitudinal axis (compare the Odontogriphus specimen in Caron et al. 2007, fig. 1B). If a genuine relationship exists between the Mount Cap microfossils and their apparent macroscopic counterparts, there is clear potential for a newly detailed understanding of the feeding apparatus in Wiwaxia and Odontogriphus. This would greatly clarify the phylogenetic significance of these pivotal early lophotrochozoans (see Caron et al. 2006, 2007; Butterfield 2006).
THE MOUNT CAP COMPARED TO OTHER BST LAGERSTÄTTEN
16 The Mount Cap Formation is notable for extending the known geographic, temporal and palaeoenvironmental scope of Burgess Shale-type preservation. It contains the most northerly occurrence of BST macrofossils in Canada, and stands apart from the cluster of localities in the southern Canadian Rockies (e.g. Collins et al. 1983; Copeland 1993; Butterfield 2000; Johnston et al. 2009; Caron et al. 2010). As well as being geographically distinct, the Mount Cap partly lies within the latest Early Cambrian (latest Series 2), an interval that contains comparatively few BST occurrences worldwide, in contrast to the dense sampling both above and below (e.g. Hagadorn 2002; Zhu et al. 2006). Furthermore, the comparatively shallow, intracratonic setting of the Mount Cap contrasts with the deeper water, shelfedge settings of the Burgess Shale and many other BST sites (e.g. Conway Morris 1989).
17 That said, BST macrofossils are comparatively rare in the Mount Cap and fall within the more recalcitrant end of the preservational spectrum, which is dominated by biomineralizing forms and heavily cuticularized body parts such as claws (anomalocaridids) and carapaces (e.g. Isoxys). Comparable remains occur, often abundantly, in the Burgess Shale and at many other localities worldwide (e.g. Hagadorn 2002). Similarly, macroscopic fecal pellets/bromalites are documented from a number of Cambrian deposits in North America, Australia and China (see Conway Morris and Robison 1988; Nedin 1999; Vannier and Chen 2005). In contrast, the Mount Cap microfossils from both outcrop and subsurface horizons are truly exceptional in terms of preservational quality and paleobiological significance.
18 Comparisons across an increasing sample of carbonaceous microfossil assemblages from Cambrian marine mudrocks (e.g. Butterfield 2008; Harvey 2010; Harvey et al. in press) suggest that the particular value of the Mount Cap lies in a combination of favourable early diagenesis and quiescent post-depositional history. For example, acid-extractable specimens from the Burgess Shale include a comparatively low diversity of animal remains (Butterfield 1990; Gostlin 2006) and rather amorphous fecal pellets (Robbins et al. 1985), and are mostly fragmentary, blackened and brittle – a reflection of their associated greenschist-grade metamorphism (Butterfield et al. 2007). In contrast, assemblages from the Middle Cambrian Kaili Formation of south China (Harvey et al. in press) and the Early Cambrian Forteau Formation of western Newfoundland (Harvey 2010, and unpublished data) are less thermally altered and contain a greater variety of recoverable microfossils, including Little Bear-type remains of chancelloriid sclerites and hyolithid helens. These specimens, however, lack the finestscale features of their Mount Cap counterparts and are notably rare, which points to constraints in early diagenesis (see Harvey et al. in press). A closer comparison to Mount Cap-type preservation is provided by the crustacean cuticles recently discovered in the Earlie/Deadwood Formation (Middle to Late Cambrian) of Saskatchewan (Harvey et al. 2010). Even so, the O-35 assemblage remains unequalled in terms of abundance and anatomical diversity.
19 Through detailed taphonomic analysis of assemblages in the Burgess Shale and elsewhere, it is becoming clear that the original expression of BST fossils was predominantly carbonaceous (e.g. Butterfield et al. 2007; Gaines et al. 2008). At most localities, however, the carbon has been secondarily reduced through metamorphism and/or oxidative weathering (Lin and Briggs 2010), with the loss of both resolvable detail and recoverable microfossils. In this light, the value of the Mount Cap lies in its exceptional and exceptionally unaltered taphonomy, which reveals the contrasting strengths and biases of macro- vs. microscopic preservation and thus a distinct perspective on Cambrian paleobiology.
We thank the Geological Survey of Canada, Calgary, for access to drillcore material. This work was supported by a NERC studentship (to THPH) and NERC grant NE/H009914/1, and by Selwyn and Sidney Sussex colleges, Cambridge.