Series
International Year of Planet Earth 6.
Biosignatures: Interpreting Evidence of the Origins and Diversity of Life
G. F. SlaterSchool of Geography and Earth Sciences, McMaster University, Hamilton, ON, Canada, L8S 4L8
gslater@mcmaster.ca
Submitted November 2008, Accepted as revised May 2009
SUMMARY
Biosignatures are molecular, mineral or isotopic patterns that can be unambiguously interpreted as evidence of life and so provide the means for us to address our most fundamental questions about the origins and evolution of life. Biosignatures of microbial life are especially important to our understanding of early Earth history, and can be recorded in magnetic mineral traces, various carbon compounds, and stable isotope ratios of many elements. These signatures, preserved in the geologic record, represent the primary means by which we gain insight into the early history of life on Earth, including the timing of the origins of life and major interactions of life with its environment, such as the oxidation of the Earth’s atmosphere. In addition, microbial biosignatures are also considered one of the most likely targets in the search for life beyond Earth. However, identifying and interpreting geochemical biosignatures of microbial life is challenging and involves careful differentiation between signatures of biological processes and those of abiological processes. Canada is playing an important role in biosig-nature research, both through the geologic record of life preserved in our ancient rocks, and through many examples of microbial life in extreme environments. The latter provide the modern understanding required to interpret the biosignature record from early Earth and perhaps one day from another planet.SOMMAIRE
Les biosignatures sont ces arrangements moléculaires, minéraux et isotopiques qui sont des preuves évidentes de l’existence de vie organique, et qui sont donc autant de moyens nous permettant de tenter de répondre aux questions fondamentales sur l’origine et l’évolution de la vie. Les biosig-natures microbiennes sont particulièrement importantes pour la compréhension des premiers stades de l’histoire de la Terre; elles sont constituées de traces de minéraux magnétiques, de composés organiques divers, ou de ratios particuliers d’isotopes stables de nombreux éléments. Ces signatures conservées dans la roche sont des indicateurs de première importance nous permettant de déchiffrer les premiers stades de l’histoire de la vie sur Terre, incluant la chronologie des origines de la vie et des grandes interactions de la vie avec l’environnement, comme l’oxydation de l’atmosphère terrestre. De plus, on considère que les biosignatures microbiennes sont l’un des indicateurs les plus probables de la présence de vie extraterrestre. Cela dit, l’identification et l’interprétation de biosignatures géochimiques microbiennes est délicate; il faut pouvoir distinguer les biosignatures de processus biologiques de celles de processus abiologiques. Le Canada joue un rôle important dans la recherche sur les biosignatures, à la fois par les traces de vie préservées dans les roches anciennes de son histoire géologique, et du fait des exemples de vie microbienne dans des environnements extrêmes de son territoire. Les recherches en milieux extrêmes nous permettent d’acquérir les connaissances nécessaires pour interpréter les biosignatures des premiers stades de l’histoire géologique de la Terre, et peut-être un jour d’une autre planète.INTRODUCTION
1 Understanding the origin and evolution of life remains one of the grand challenges of scientific inquiry. Our interest in this question is further increased by tantalizing glimpses of other solar system bodies where we can imagine life may exist or may have existed, such as Mars, Europa and Enceladus (McKay 2008). Furthermore, the recent detection of planets orbiting distant stars (Lovis et al. 2006) conjures the image of a universe where we may not be alone. However, while we may hope that new research into these extra-terrestrial systems may answer these fundamental questions, there remains only one planet on which we are certain that life arose and has existed, the planet Earth. Thus, the study of the history of life on Earth is at once the study of our own history and the only basis we have to build our knowledge of what represents evidence of life. The study of the history of life on Earth has a second important relevance to society today and that is an awareness of the response of life to changes in environmental conditions, particularly climate. As evidence increases that human activities are impacting the Earth’s environment, learning the impacts of such changes on life, from the micro to the macro scale, is more pressing than ever.
2 Although our anthropocentric view may suggest otherwise, the dominant form of life on Earth today and throughout the geologic record is microbial life. Microbial life is also the most likely form of life to exist elsewhere in the universe. We are increasingly recognizing the crucial role played by microbes in Earth systems, from cycling of nutrients (Falkowski 1997; Canfield and Raiswell 1999; Falkowski et al. 2008) and organic carbon (Petsch et al. 2001), to weathering of rock (Roberts and Bennett 2004). Such processes have the potential to leave recognizable signatures (i.e. biosignatures) in minerals, organic compounds and/or isotopic ratios, and these signatures can be preserved in the geologic record. By understanding the metabolic capabilities, impacts and biosignatures of microbial life, we can gain insight into how the Earth functions, and into the history of life on Earth. Furthermore, this insight forms the only basis we have for the search for life on other astronomical bodies, whether this involves looking for specific signatures that have been observed on Earth, or for biogeo-chemical patterns created by a type of life unlike that known on Earth.
3 The goals of this paper are twofold: 1) to highlight recent research into microbial biosignatures in the geologic record and in modern analogue systems; and ii) to describe the contributions this research has made and the questions it has raised about evidence for life on ancient Earth and beyond Earth. In particular, this paper focuses on several key issues being faced today and the role that Canada and Canadian scientists are playing in addressing them. These issues include: i) the potential for abiogenic production of organic matter and the significance of this to our understanding of the timing of life’s origins; ii) the timing of oxidation of earth’s atmosphere; and iii) the continued expansion of our understanding of the capabilities and limits of microbial life. As will be evident from the examples discussed herein, there may be no single signature that can serve as unambiguous evidence of life; instead, research is needed to bring together multiple lines of evidence in order to convincingly demonstrate the presence and impacts of life.
TYPES OF BIOSIGNATURES
Biosignatures and Abiosignatures
4 In any discussion of biosignatures, one of the first and most pressing questions is how we can differentiate between biosignatures and ‘abiosignatures’. In this usage, the term ‘abiosig-natures’ refers to a signature derived from an abiotic (i.e. chemical or physical) process. Only by demonstrating that a given signature can only be produced by biological activity can we conclude that it represents evidence of life. Therefore, in order to interpret a mineral, organic or isotopic signature as a biosignature, we must also be certain that it cannot be produced by abiotic processes, i.e. that it does not mimic a biosignature. It is worth noting that such interpretations should always be made with the awareness that as our understanding increases, what was once assumed to be a biosig-nature may cease to be considered so in light of new information.
Mineral Biosignatures
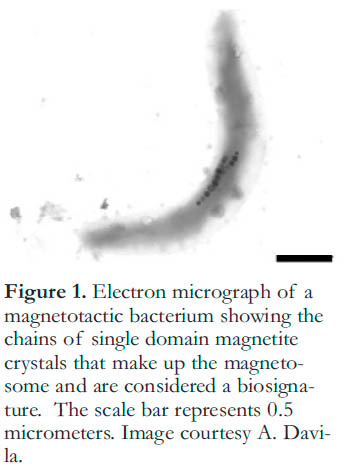
5 The stability of minerals over long periods of time and a wide range of conditions means that they have the potential to preserve biosignatures. Chains of single-domain magnetite crystals produced by magnetotactic bacteria represent one example of a mineral biosignature that has received attention recently (Sukumaran 2005). These bacteria form magnetite crystals as part of a magnetosome (Fig. 1), which allows them to orient their movement and stay at the geochemical interface between oxic and anoxic conditions in the sediments where their optimal growth conditions occur (Kopp and Kirshvink 2008). These magnetofossils are present in the geologic record back to the Cretaceous, and potentially to the Archean (Chang and Kirshvink 1989; Kopp and Kirshvink 2008). However, recognition of these crystals as biosignatures requires careful assessment in order to differentiate them from abiotically produced crystals (Kopp and Kirshvink 2008). The observation of magnetite crystals has been proposed as evidence for life in the meteorite ALH84001 (Thomas-Keprta et al. 2002), but this argument has yet to be accepted as definitive. Ongoing study seeks to further define the biogenic characteristics of magnetofossils (Kopp and Kirshvink 2008) and to add other lines of evidence to support their biogenicity, such as isotopic ratios (Mandernack et al. 1999).
Figure 1. Electron micrograph of a magnetotactic bacterium showing the chains of single domain magnetite crystals that make up the magneto-some and are considered a biosignature. The scale bar represents 0.5 micrometers. Image courtesy A. Davila.Organic Biosignatures
6 Because life on Earth is based on carbon chemistry, organic compounds have a great deal of potential as biosig-natures. In fact, it was organic geo-chemists who originally used the term “biological marker compounds” or “biomarkers” to describe organic compounds that could survive over the long periods and potential diagenetic alterations associated with geological processing, and still retain an ability to be related to a specific biological origin (Eglinton et al. 1964). Determination of effective biomarker compounds requires that their production by modern organisms be characterized. For instance, one class of biomarker compounds produced by bacteria, the hopanoids, is found throughout the geologic record (Summons et al. 1999; Brocks et al. 2003), and one hopanoid class in particular, the 2-methyl hopanoids, have been used to illuminate the role of cyanobacteria in the rise of photosynthesis (Summons et al. 1999). However, as with all biosignatures, understanding how these signatures are synthesized by modern organisms is crucial to their interpretation. Recent research in measuring the production of bacteriohopanpolyols, particularly 2-methyl bacteriohopanpolyol in modern cyanobacteria, has found that, although they are produced by the majority of species, the most prolific marine picocyanobacteria do not produce them, whereas two prolific marine nitrogen-fixing species do (Talbot et al. 2008). This new information does not call into question the utility of these compounds as a biomarker of microbial life in general, but it does raise important questions regarding the extent to which they can be used as a marker of specific species.
7 Because of the complexity of organic chemistry and its fundamental role in life as we know it, organic bio-markers are of great interest as a focus of the search for life on other astronomic bodies. The state of knowledge concerning organic biosignatures and their application was recently summarized by Summons et al. (2008), who describe a series of general patterns of measurable molecular biosignatures that may be used to identify life. These patterns include i) molecular distributions that display a preference for one specific spatial arrangement of atoms (known as a stereoisomer) over another; ii) molecules constructed of repeating constitutional sub-units (e.g. proteins, which are constructed of amino acids); and iii) uneven distributions of structurally related compounds (biological systems will often produce very large amounts of a small sub-set of potential compounds rather than a continuum of compounds built from smaller sub-units). These general patterns have been developed from our observations of life on Earth, but by focusing on general properties of biological compounds we may be able to develop a framework that will also enable us to recognize signatures of life that is based on different chemistries.
Isotopic Biosignatures
8 Isotopic biosignatures are changes in stable isotope ratios that can be directly related to biological activity. Specific isotopic distribution patterns are also included in the Summons et al. (2008) description of organic biosignatures. However, isotopic biosignatures can exist for nearly all elements because, with a few exceptions, all elements comprise an array of stable isotopes. The term isotope comes from the Greek meaning ‘same place’ because all isotopes of a given element occupy the same place in the periodic table. This means that isotopes of an element all have the same basic chemical properties, the primary difference being their atomic mass, which varies according to the number of neutrons in the nucleus. In radioactive isotopes the nucleus is unstable and decays via one of several mechanisms. The extent of radioactive decay is the basis for much of our dating of geological materials (Faure 1986) and has therefore contributed a great deal to our understanding of the timing of biosignatures observed over the history of life on Earth. However, because the abundance of radioactive isotopes is constantly changing, they cannot preserve a direct isotopic biosignature over long periods of time. In contrast, stable isotopes, isotopes in which the mass difference does not cause the atom to be unstable, do not change over time and can therefore preserve a biosignature (Faure 1986). For example, carbon has two stable isotopes, carbon 12 and carbon 13, which compose approximately 98.9% and 1.1%, respectively, of any mass of carbon on Earth. For any sample of carbon-bearing molecules, we can determine the precise ratio of the isotopes of carbon, and express it in delta notation as follows: δ13C = (Rsample – Rstandard)/Rstandard * 1000 in units of per mil (%) (1),
where R is the ratio 13C/12C. This expression for isotopic ratios can be generalized to any atom by replacing 13C and 12C with the isotopes of interest. Note that by convention in geo-chemistry, the heavier (and less abundant) isotope is divided by the lighter (more abundant) isotope. In our example, 13C has the same chemical properties as 12C, but is one atomic mass unit heavier, hence when it is involved in processes in which mass plays a factor, it behaves in different ways. These differences in behaviour result in isotopic ‘fractionation’ of the ratio of 13C to 12C in a sample. This isotopic fractionation can be described by a fractionation factor, which is characteristic for the process:α = 1000 + δ13CA/1000 + δ13CB (2),
where A and B are two components or phases of a chemical process, such as the reactant and product, or gas phase and aqueous phase. Both the stable isotopic ratios and the fractionation factors that change them can be characteristic for different processes and have been used as biosignatures of life on Earth.
Application of Biosignatures
9 Biosignature research uses all of the foregoing types of signatures, ideally in combination, to generate an interpretation of the history of life. Developing this interpretation is contingent on studies focused in two areas. The original focus of biosignature research was the acquisition of samples from the entire geologic record that could be used to provide a history of life on Earth. Canada is a logical location for this type of research because our rocks record a broad swath of geologic history, including some of the oldest rocks known. The second focus of the research requires study of modern analogues to aid in the interpretation of the geological samples. In other words, to interpret the signatures observed in the rock record, we must have some grasp of the processes that created those signatures. By using examples of modern systems that are analogous to past systems, we can explain the mechanisms by which biosignatures form, we can differentiate between biosignatures and abiosignatures, and we can determine what these signatures tell us about life and/or its environment at the time. The remainder of this paper highlights some of the current research on both of these fronts, and how it is contributing to our understanding of the origins and evolution of life.
BIOSIGNATURES IN THE GEOLOGIC RECORD
Biogenic and Abiogenic Hydrocarbons
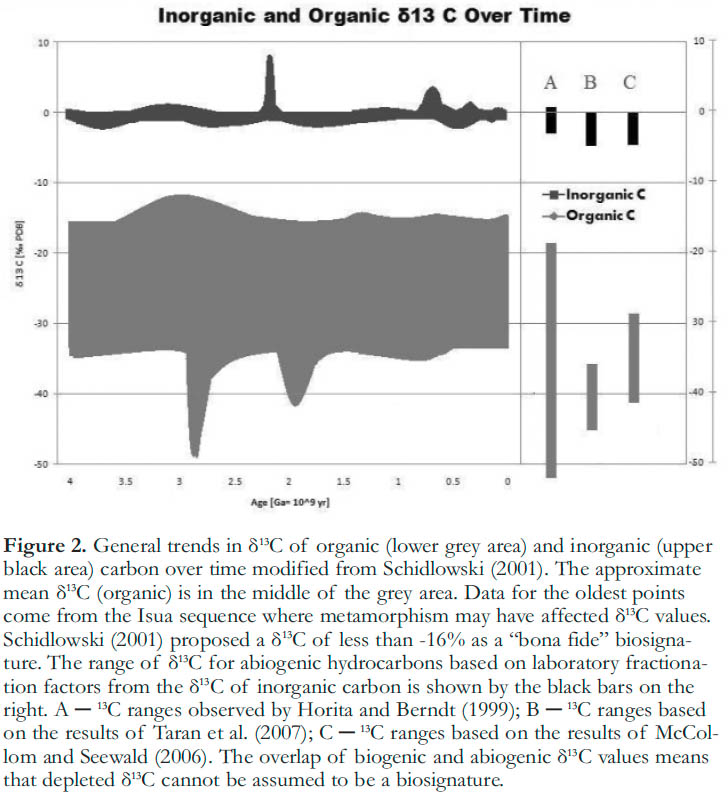
10 One of the most fundamental unanswered questions concerning life on Earth is the timing of its origin. Because Canada hosts some of the oldest rocks on Earth, e.g. the Nuvvuagittuq greenstone belt in Northern Quebec (Cates and Mojzsis 2007; O’Neil et al. 2008), this country is well positioned to contribute samples that can provide unique insights into this question. Interpreting signatures in these ancient samples as evidence of early life on Earth hinges on the issue of biosignatures versus abiosignatures. In general, the geologic record shows that organic carbon is isotopically depleted in 13C compared to inorganic carbon. Schidlowski (2001) summarized the data on organic and inorganic isotopes (Fig. 2), and stated that isotopically depleted organic carbon (δ13C <-16%) is a bona fide biological signature. Furthermore, it has been widely stated that isotopic depletion in 13C is a result of biological activity. However, this conclusion is now being reconsidered in the light of recent research demonstrating that abiogenic hydrocarbon formation via chemical reactions between water, dissolved inorganic carbon and minerals is not only capable of producing reduced organic carbon, but that this organic carbon is strongly isotopically depleted with respect to its inorganic source. Methane with isotopic compositions ranging from -19 to -53% relative to initial isotopic compositions of -4.1% for the carbon source, was described by Horita and Berndt (1999), and δ13C values ranging from -40 to -50% for hydrocarbons produced via the Fischer-Tropsch synthesis, corresponding to fractionations of 38%, were reported by Taran et al. (2007). McCollom and Seewald (2006) also documented the production of strongly depleted hydrocarbons (δ13C of -44 to -50%), corresponding to similar fractionations of 31-36%, during abiogenic reactions under hydrothermal conditions (250ºC, 325 bar) (Fig. 2). Comparison of these ranges with those observed in the geologic record demonstrates that isotopic depletion of 13C in organic matter cannot be considered an unambiguous biosignature. This point is emphasized by recent evidence of abiogenic hydrocarbons in natural systems, based on unique 13C and 2H signatures in hydrocarbons from Kidd Creek mine in Northern Ontario (Sherwood Lollar et al. 2002). Subsequently, evidence of abiogenic hydrocarbons has also been found in the Lost City hydrothermal system near the Mid Atlantic Ridge (Proskurowski et al. 2008).
Figure 2. General trends in δ13C of organic (lower grey area) and inorganic (upper black area) carbon over time modified from Schidlowski (2001). The approximate mean δ13C (organic) is in the middle of the grey area. Data for the oldest points come from the Isua sequence where metamorphism may have affected δ13C values. Schidlowski (2001) proposed a δ13C of less than -16% as a “bona fide” biosignature. The range of δ13C for abiogenic hydrocarbons based on laboratory fractionation factors from the δ13C of inorganic carbon is shown by the black bars on the right. A ─ 13C ranges observed by Horita and Berndt (1999); B ─ 13C ranges based on the results of Taran et al. (2007); C ─ 13C ranges based on the results of McCollom and Seewald (2006). The overlap of biogenic and abiogenic δ13C values means that depleted δ13C cannot be assumed to be a biosignature.
Display large image of Figure 2
11 The observation of abiogenic production of isotopically depleted hydrocarbons, both in laboratory studies and in the field, raises significant questions regarding the conclusion of Schidlowski (2001) and others that isotopically depleted organic carbon observed in ancient rocks is a biosignature. Since the presence of hydrocarbons is a necessary precondition for life, and since hydrocarbons formed by abiogenic processes may not be isotopically distinguishable from biogenic hydrocarbons, it is extremely difficult to assess whether the isotopic depletions observed in ancient rocks are indeed a biosignature that records the origins of life on Earth. It follows that observation of isotopically depleted organic matter on other planets or solar system bodies can not be unambiguously interpreted as a biosignature. To overcome this limitation, we need to find multiple converging lines of evidence that demonstrate the presence and activities of life.
Biosignatures in the Geologic Record: Oxygenation of the Atmosphere
12 Another example of how our understanding is changing with the acquisition of new information has to do with oxygenation of the atmosphere. The rise of atmospheric oxygen, often referred to as the Great Oxidation Event (GOE; Canfield 1998), is one of the most significant events in the history of life on Earth; this event represents one of the greatest impacts that life has had on the global environment, and on all subsequent life. In this instance, microbial life drastically affected its environment and changed its properties, making the Earth’s atmosphere an ‘extreme’ environment to the anaerobic organisms then living under the anoxic conditions that prevailed. However, it also created the dis-equilibrium between reduced carbon and free oxygen that provided the thermodynamic energy basis for aerobic life on Earth, and is arguably a requirement for the development of terrestrial macrobiota. This disequilibrium is accepted as a biosignature of photo-synthetic life on Earth; hence, spectroscopic evidence of oxygen in the atmospheres of new planets being discovered around distant stars is a potential biosignature. However, the extent to which atmospheric oxygen can be considered a biosignature is defined by our ability to be certain that it could not also be created by abiotic processes. Ongoing research into the oxygenation of Earth’s atmosphere is one approach that contributes to our ability to answer this question.
13 Because of the crucial role it has played in the development of life on Earth, the occurrence and timing of the increase in concentration of oxygen in the atmosphere is the subject of multiple lines of current research, including new approaches in isotopic analysis. A prime example is the use of Mass-independent Isotopic Fractionation (MIF) of sulphur as a tracer of the sulfur cycle, and, through geo-chemical connection, as a measure of the abundance of oxygen in the atmosphere (Farquhar et al. 2000). MIF occurs by significantly different processes than the mass dependent fractionation described in the previous section. As the name suggests, it is not related to the mass difference between isotopes, but to other atomic properties. The specific mechanisms of MIF are the subject of ongoing research (Theimens 2006). However, using the MIF of sulphur 33 (Δ33S), Farquhar et al. (2000) have demonstrated significant changes in the Earth’s atmospheric sulphur cycle (Fig. 3), which they have used to argue that a massive increase in atmospheric oxygen occurred at 2450 Ma.
Figure 3. Ranges of Δ33S (%) (Farquhar and Wing 2003) and δ56Fe (%) (Rouxel et al. 2005) over time. The rise of atmospheric oxygen is demonstrated by dramatic decreases in the ranges observed in both isotopic systems.
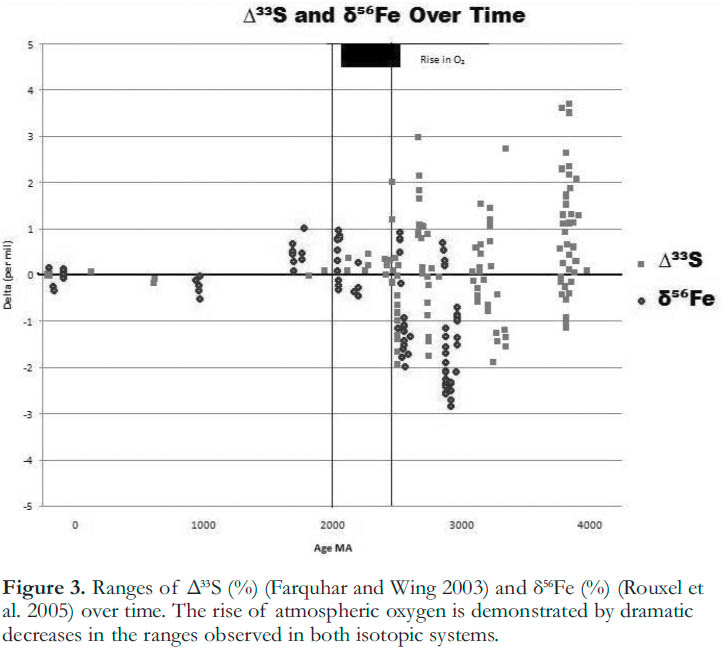
Display large image of Figure 3
14 Rouxel et al. (2005) have applied new approaches in iron isotope analysis to the same question and have shown that the range in iron isotopic compositions was drastically reduced within the same timeframe as that proposed by the sulphur MIF signal for the GOE (Fig. 3). The decrease in negative δ56Fe signatures observed across the GOE is interpreted by Rouxel et al. (2005) as the result of the oxidation and precipitation of large amounts of iron. The continued presence of positive δ56Fe during the GOE is argued to be evidence of ongoing redox stratification in the ocean at this time; i.e. the deep ocean was not fully oxic. The timing of the changes in δ56Fe coincides very closely with the changes in Δ33S, indicating that these two independent isotope systems are recording evidence of the same event.
15 The timeframe for these isotopic excursions is concurrent with the formation of large deposits known as banded iron formations. Banded iron formations are alternating layers of iron oxide-rich and silicate-rich minerals that range in thickness from meter to sub-millimeter scales and range in age from ca. 2.7 to 1.9 Ga. They have long been related to the oxygenation of the Earth’s atmosphere, but recent research is investigating new roles that microbial activity likely played at several steps in the formation of these structures. Konhauser et al. (2005) developed a model indicating that heterotrophic (i.e. requiring organic compounds of C and N for nourishment) microbial metabolism was an important component in their genesis, whereas Johnson et al. (2008) applied iron isotope systematics to suggest a specific role for microbial iron reduction. Although our knowledge of the complex processes that led to the production of banded iron formations is by no means complete, by bringing together multiple lines of evidence from multiple biosignatures, we are getting much closer to a conceptual model of what occurred at this crucial time in Earth history.
THE SEARCH FOR NEW GEOLOGIC BIOSIGNATURES
Microbe – Rock Interactions
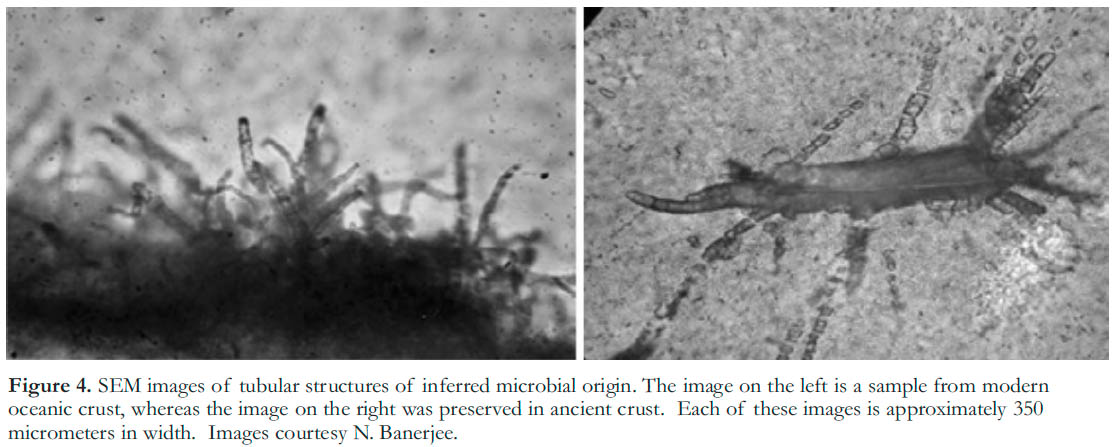
16 Microbial impacts on the environment can also occur at much smaller scales than the oxidation of the atmosphere, and can be far more difficult to recognize. However, these smaller-scale effects have the potential to engender new biosignatures that may be preserved over long geologic timescales. For example, Furnes et al. (2004) reported the occurrence of (microbial) microtubules in volcanic glasses from the Barberton Greenstone Belt in South Africa and in samples from modern oceanic crust, that have been interpreted as a new biosignature (Fig. 4). Although modern examples of surface etching by microorganisms can be observed in the laboratory, the timescales required to form such extended tubules are too great to allow experimental testing. Without the ability to directly demonstrate microbial creation of these tubules, researchers are bringing together multiple lines of evidence, including element mapping, carbon isotopes, scanning transmission x-ray microscopy (STXM), transmission electron microscopy (TEM), molecular genetic techniques, and dating methods (Banerjee et al. 2006, 2007; Benzerara et al. 2007). These techniques are being applied to samples of pillow lava from South Africa, Australia and the Abitibi Greenstone Belt in Canada, to determine their origin and whether the tubules constitute a biosignature.
Figure 4. SEM images of tubular structures of inferred microbial origin. The image on the left is a sample from modern oceanic crust, whereas the image on the right was preserved in ancient crust. Each of these images is approximately 350 micrometers in width. Images courtesy N. Banerjee.Modern Microbial Biosignatures
17 To provide the foundation needed to interpret biosignatures in the geologic record, research characterizing modern biosignatures is required. This research must address a wide range of questions/conditions, since much of life’s diversity arises from the ability of microbial life to evolve metabolic capabilities that allow it to inhabit almost every known environment on Earth. Microbes have been found to thrive in extreme ranges of temperature, salinity, pH, radiation, pressure, water activity and oxygen availability. Furthermore, they are extremely metabolically diverse and can obtain energy from a wide array of sources, both autotrophically and heterotrophically. If research succeeds in characterizing the potential biosignatures that may be associated with this wide range of adaptability and metabolic flexibility, the information can then be used as a modern template by which either ancient terrestrial or extraterrestrial biosignatures are interpreted.
18 The range of natural environments within Canada provides important opportunities to investigate the formation of biosignatures, the mechanisms by which life creates them, and fundamental aspects of the limits of life. This environmental diversity is used to advantage by the Canadian Space Agency’s ‘Canadian Analogue Research Network’ (CARN), which supports a wide range of research at sites across Canada, including the three main sites at the McGill Arctic Research Station on Axel Heiberg Island, the Houghton Mars Project on Devon Island, and the Pavilion Lake Research Project in British Columbia. Research conducted at these sites focuses on how life survives in extreme environments and the signatures it leaves behind. Documentation of microbial biosignatures generated at these sites will increase our knowledge of the capabilities of life and allow us to interpret evidence of life not only from Earth’s geologic record, but also in samples from Mars and elsewhere.
Modern Biosignatures: Life in Extreme Environments
Microbialites and Microbial Mats
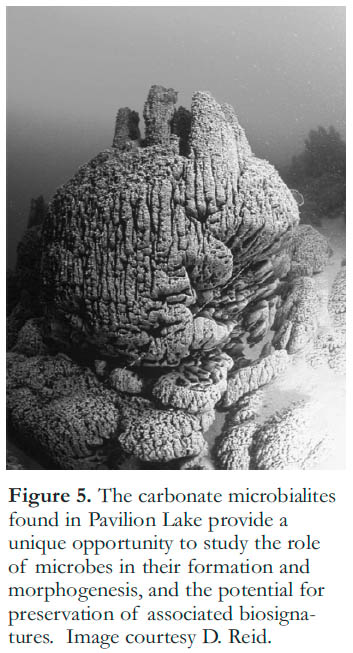
19 The interaction between rocks and microbial communities is the primary focus of the collaborative work led by Canadians as part of the Pavilion Lake Research Project in Pavilion Lake, British Columbia. The large calcium carbonate microbialites at Pavilion Lake (Laval et al. 2000; Fig. 5) are being investigated to determine the role of biology in their formation, in their unique morphologies, and in their potential for preservation of associated biosignatures. The term microbialite refers to organo-sedimentary structures that arise from interactions between microbes and geologic environments (Burne and Moore 1987). Understanding the formation of microbialites will provide insight that may contribute to interpreting the early record of life preserved in other organo-sedimentary structures such as stromatolites (Grotzinger and Knoll 1999). Research also aims to compare these microbialites to the cyanobacterially dominated microbial mats present in the saline, alkaline lakes of the Cariboo plateau (Renaut and Stead 1990; Renaut 1993). These evaporitic systems, which have high salinities (>sea-water), high alkalinities, and pHs ranging from neutral to over 10, provide a natural laboratory to investigate the microbial metabolisms, processes and signatures of microbial mat systems. This knowledge can then be used as an analogue for microbial mat systems in Earth’s geologic record or on the surface of Mars, where water might have existed (Squyres et al. 2004), possibly in association with high solute concentrations (Fairen et al. 2009).
Figure 5. The carbonate microbialites found in Pavilion Lake provide a unique opportunity to study the role of microbes in their formation and morphogenesis, and the potential for preservation of associated biosignatures. Image courtesy D. Reid.Microbes in the Deep Subsurface
20 Microbe-rock interactions also occur in the extreme environment of the deep terrestrial subsurface. Microbial life in the deep subsurface is the most likely form of life to have existed recently on Mars (Mancinelli 2000) and has been hypothesized as a potential location for the origin of life on Earth (Onstott et al. 1998; Whitman 1998). Discovering the dynamics of subsurface life, the timescales over which it occurs and the signatures it leaves behind, represents another avenue of biosignature research. For example, investigations in the deep, crystalline rocks of the terrestrial subsurface has shown evidence of methane generated by the reduction of inorganic carbon (Chappelle et al. 2002; Ward et al. 2004), and has resulted in the observation of several unique organisms whose metabolic capabilities and modes of survival are just starting to be revealed (Chivan et al. 2008; Wanger et al. 2008). Further research to increase our understanding of microbial life in these extreme systems is ongoing at several sites, including sites in the Canadian Shield such as Kidd Creek mine. This research into deep microbial life also has economic implications: Investigation of the biodegradation of petroleum deposits in sedimentary subsurface environments is being undertaken to learn how microbial metabolic activities affect petroleum deposits over geologic timescales (Bennett et al. 2006).
Cryoenvironments: Arctic Research
21 Researchers at the McGill Arctic Research station and other locations in the Canadian Arctic have been investigating microbes capable of living under extremes of cold and limited availability of water. Conditions in Earth’s polar desert and permafrost are one of the best analogues for the cold, permafrost conditions that exist near the poles of Mars (Steven et al. 2006). Recent research has identified a wide range of organisms living in the permafrost (Steven et al. 2007a) including a novel aerobic, spore-forming bacterium (Steven et al. 2008). These organisms are biogeochemically active and use a range of metabolisms from aerobic (Steven et al. 2008), to anaerobic (Rivkina et al. 1998), to methanogenic. Furthermore, these organisms are metabolically active below the freezing point of water both in laboratory (Rivkina et al. 2000) and field studies (Steven et al. 2007b). Extensive research continues in order to better comprehend the mechanisms by which these organisms survive, and to identify the biosignatures that may be preserved in this unique environment.
Viruses
22 It is increasingly apparent that any study of the functioning of microbial communities must also investigate viruses. Viruses are particularly interesting when considering the search for life on other planets because, while they clearly have many attributes of life, they fail certain assessments that are thought to be fundamental to life, such as the ability to self-replicate. Viruses certainly do replicate, but only via interactions with a host organism. We are just beginning to understand the huge impact that viruses have in the modern environment, let alone the crucial role they may have played in the past. The prevalence of viral infections and the transfer of genetic material that this involves means that viruses have possibly played a major role in the evolution and diversification of life and the global ecosystem (Suttle 2007). Research has shown that the number of viruses in the marine system is far above expectations, and that these viruses are highly active in the marine carbon cycle (Suttle 2007). This activity not only has significant implications for the global carbon cycle, but it may also play a significant role in the transfer of genetic material between microorganisms and therefore in the evolution of new metabolic capabilities (Suttle 2005).
CONCLUSIONS
23 Without doubt the research discussed herein touches only a small component of the extensive work being done to gain insight into life’s biosignatures. The ongoing studies of modern microbial systems in extreme environments provide the foundations for interpreting the biosignatures of life on the early Earth, and also the fundamental knowledge required to interpret the results of the search for life on other planets. It is becoming evident that this is by no means a simple task, and our understanding is certainly not complete. But only by continuing to investigate biosignatures in the geologic record, and the modern analogues that allow us to interpret them, will we be able to define the differences between biosignatures and abiosignatures. Canada is contributing a great deal to biosignature research, both from the perspective of the geologic and modern environments represented in Canada, and via the ongoing studies at Canadian institutions. As this work progresses and we elucidate the nature and implications of biosignatures, Canada and Canadian researchers have the potential to contribute much more toward understanding the history and diversity of life on Earth, and in determining whether life may have existed elsewhere in the solar system.
ACKNOWLEDGEMENTS
Thank you to all of my collaborators and colleagues with whom I have discussed these very interesting issues over the past few years. I look forward to the progress that results from our cumulative efforts. Thank you specifically to A. Davila, N. Banerjee and D. Reid for the images. And particular thanks to P. Morrill, E. Van der Flier-Keller, an anonymous reviewer and editors Jim Teller and Reg Wilson for their insightful feedback and input that contributed greatly to improving this manuscript.REFERENCES
Banerjee, N.R., Furnes, H., Muehlenbacks, K., Staudigel, H., and de Wit, M., 2006, Preservation of 3.4-3.5 Ga microbial biomarkers in pillow lavas and hyaloclastites from the Barberton Greenstone Belt, South Africa: Earth and Planetary Science Letters, v. 241, p. 707-722.
Banerjee, N.R., Simonetti, A., Furnes, H., Muehlenbachs, K., Staudigel, H., Heaman, L., and Van Kranendonk, M.J., 2007, Direct dating of Archean microbial ichnofossils: Geology, v. 35, p. 487-490.
Bennett, B., Fustic, M., Farrimond, P., Huang, H., and Larter, S.R., 2006, 25-Norhopanes: Formation during biodegradation of petroleum in the subsurface: Organic Geochemistry, v. 37, p. 787-797.
Benzerara, K., Menguy, N., Banerjee, N.R., Tyliszczak, T., Brown, G.E., and Guyot, F., 2007, Alternation of submarine basaltic glass from the Ontong Java Plateau: A STXM and TEM study: Earth and Planetary Science Letters, v. 260, p. 187-200.
Brocks, J.J., Buick, R., Logan, G.A., Summons, R.E., 2003, Composition and synergy of molecular fossils from the 2.78-2.45 billiion year old Mount Bruce supergroup, Pilbara Craton, Western Australia: Geochemica et Cosmochemica Acta, v. 67, p. 4289-4319.
Burne, R.V., and Moore, L.S., 1987, Microbialites: Organosedimentary deposits of benthic microbial communtities: Palaios, v. 2, p. 241-254.
Canfield, D.E., 1998, A new model for Proterozoic ocean chemistry: Nature, v. 396, p. 450-453.
Canfield, D.E., and Raiswell, R., 1999, The evolution of the sulfur cycle: American Journal of Science, v. 299, p. 697-723.
Cates, N.L., and Mojzsis, S.J., 2007, Pre-3750 Ma supracrustal rocks from the Nuvvagittuq supracrustal belt, northern Quebec: Earth and Planetary Science Letters, v. 255, p. 9-21.
Chang, S.B.R., and Kirshvink, J.L., 1989, Magnetofossils, the magnetization of sediments and the evolution of magnetite biomineralization: Annual Reveiws in Earth and Planetary Science, v. 17, p. 169-195.
Chappelle, F.H., O’Neill, K., Bradley, P.M., Methe, B.A., Ciufo, S.A., Knobel, L.L., and Lovley, D.R., 2002, A hydrogen-based subsurface microbial community dominated by methanogens: Nature, v. 415, p. 312-315.
Chivan, D., Brodie, E.L., Alm, E.J., Culley, D.E., Dehal, P.S., DeSantis, T.Z., Gihring, T.M., Lapidus, A., Lin, L.H., Lowry, S.R., Moser, D.P., Richardson, P.M., Southam, G., Wanger, G., Pratt, L., Andersen, G.L., Hazen, T.C., Brockman, F.J., Arkin, A.P., and Onstott, T.C., 2008, Environmental genomics reveals a single-species ecosystem deep within Earth: Science, v. 322, p. 275-278.
Eglinton, G., Scott, P.M., Belsky, T., Burlingame, A.L., and Calvin, M., 1964, Hydrocarbons of biological origins from a one-billion year old sediment: Science, v. 145, p. 263-264.
Fairen, A.G., Davila, A.F., Gago-Duport, L., Amils, R., and McKay, C.P., 2009, Stability against freezing of aqueous solutions on early Mars: Nature, v. 459, p. 401-404.
Falkowski, P.G., 1997, Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean: Nature, v. 385, p. 272-275.
Falkowski, P.G., Fenchel, T., and Delong, E.F., 2008, The microbial engines that drive Earth’s biogeochemical cycles: Science, v. 320, p. 1034-1039.
Farquhar, J., and Wing, B., 2003, Multiple sulfur isotopes and the evolution of the atmosphere: Earth and Planetary Science Letters, v. 213, p. 1-13.
Farquhar, J., Bao, H., and Thiemens, M., 2000, Atmospheric influence of Earth’s earliest sulfur cycle: Science, v. 289, p. 756-758.
Faure, G. 1986. Principles of Isotope Geology. New York, John Wiley and Sons, 529 p.
Furnes, H., Banerjee, N.R., Muehlenbachs, K., Staudigel, H., and de Wit, M., 2004, Early life recorded in Archean pillow lavas: Science, v. 304, p. 578-581.
Grotzinger, J.P., and Knoll, A.H., 1999, Stromatolites in Precambrian carbonates: Evolutionary mileposts or environmental dipsticks?: Annual Reviews in Earth and Planetary Science, v. 27, p. 313-358.
Horita, J., and Berndt, M.E., 1999, Abiogenic methane formation and isotopic fractionation under hydrothermal conditions: Science, v. 285, p. 1055-1057.
Johnson, C.M., Beard, B.L., Kliein, C., Beukes, N.J., and Roden, E.E., 2008, Iron isotopes constrain biologic and abiologic processes in banded iron formation genesis: Geochemica et Cosmochemica Acta, v. 72, p. 151-169.
Konhauser, K.O., Newman, D.K., and Kappler, A., 2005, The potential significance of microbial Fe(III) reduction during deposition of Precambrian banded iron formations: Geobiology, v. 3, p. 167-177.
Kopp, R.E., and Kirshvink, J.L., 2008, The identification and biogeochemical interpretation of fossil magnetotatic bacteria: Earth Science Reviews, v. 86, p. 42-61.
Laval, B., Cady, S.L., Pollack, J.C., Mackay, C.P., Bird, J.S., Grotzinger, J.P., Ford, D.C., and Bohm, H.R., 2000, Modern freshwater microbialte analogues for ancient dendritic reef structures: Nature, v. 407, p. 626-629.
Lovis, C., Mayor, M., Pepe, F., Alibert, Y., Benz, W., Bouchy, F., Correia, A.C.M., Laskar, J., Mordasini, C., Queloz, D., Santos, N.C., Udry, S., Bertaux, J., and Sivan, J.-P., 2006, An extrasolar planetary system with three Neptune-mass planets: Nature, v. 441, p. 305-309.
Mancinelli, R.L., 2000, Assessing the Martian deep subsurface to search for life: Planetary and Space Science, v. 48, p. 1035-1042.
Mandernack, K.W., Bazylinski, D.A., Shanks, W.C., and Bullen, T.D., 1999, Oxygen and irons isotope studies of magnetite produced by magnetotactic bacteria: Science, v. 285, p. 1892-1896.
McCollom, T.M. and Seewald, J.S., 2006, Carbon isotope composition of organic compounds produced by abiotic synthesis under hydrothermal conditions: Earth and Planetary Science Letters, v. 243, p. 74-84.
McKay, C.P., 2008, An approach to searching for life on Mars, Europa and Enceladus: Space Science Reviews, v. 135, p. 49-54.
O’Neil, J., Carlson, R.W., Francis, D., and Stevenson, R.K., 2008, Neodymium-142 evidence for Hadean mafic crust: Science, v. 321, p. 1828-1831.
Onstott, T.C., Phelps, T.J., Kieft, T., Col-well, F.S., Balkwill, D.L., Fredrickson, J.K. and Brockman, F.J., 1998, A global perspective on the microbial abundance and activity in the deep subsur-face, in Seckbach, J., ed., Enigmatic Microorganisms and Life in Extreme Environments: Kluwer Publishing, Boston, p. 489-499.
Petsch, S.T., Eglinton, T.I., and Edwards, K.J., 2001, 14C-dead living biomass: Evidence for microbial assimilation of ancient organic carbon during shale weathering: Science, v. 292, p. 1127-1131.
Proskurowski, G., Lilley, M.D., Seewald, J.S., Fruh-Green, G.L., Olson, E.J., Lupton, J.E., Sylva, S., and Kelley, D.S., 2008, Abiogenic hydrocarbon production at Lost City hydrothermal field: Science, v. 319, p. 604-607.
Renaut, R.W., 1993, Morphology, distribution, and preservation potential of microbial mats in the hydromagnesitemagnesite playas of the Cariboo Plateau, British Columbia, Canada: Hydrobiologia, v. 267, p. 75-98.
Renaut, R. and.Stead, D., 1990, Recent magnestie-hydromagnesite sedimentation in Playa basins of the Cariboo plateau: British Columbia Geological Survey Branch, Geological Field Work, Paper 1991-1, p. 279-288.
Rivkina, E., Gilichinsky, D., Wagener, S., Tiedje, J., and McGrath, J., 1998, Biogeochemical activity of anaerobic microorganisms from buried permafrost sediments: Geomicrobiology Journal, v. 15, p. 187-193.
Rivkina, E., Friedmann, E.I., McKay, C.P., and Gilichinsky, D., 2000, Metabolic activity of permafrost bacterial below the freezing point: Applied and Environmental Microbiology, v. 66, p. 3230-3233.
Roberts, J.R., and Bennett, P.C., 2004, Mineral stimulation of subsurface microorganisms: release of limiting nutrients from silicates: Chemical Geology, v. 203, p. 91-108.
Rouxel, O.J., Bekker, A., and Edwards, K.J., 2005, Iron isotope constraints on the Archean and Paleoproterozoic ocean redox state: Science, v. 307, p. 1088-1091.
Schidlowski, M., 2001, Carbon isotopes as biogeochemical recorders of life over 3.8 Ga of Earth history: Evolution of a concept: Precambrian Research, v. 106, p. 117-134.
Sherwood Lollar, B., Westgate, T., Ward, J.A., Slater, G.F., and Lacrampe-Couloume, G., 2002, Abiogenic formation of alkanes in the Earth’s crust as a minor source for global hydrocarbon reservoirs: Nature, v. 416, p. 522-524.
Squyres, S.W., Grotzinger, J.P., Arvidson, R.E., Bell, J.F., Calvin, W., Christensen, P.R., Clark, B.C., Crisp, J.A., Farrand, W.H., Herkenhof, K.E., Johnson, J.R., Klingelhofer, G., Knoll, A.H., McLennan, S.M., McSween Jr., H.Y., Morris, R.V., Rice Jr., J.W., Reider, R., and Sonderblom, L.A., 2004, In situ evidence for an ancient aqueous environment at Meridiani Planum, Mars: Science, v. 306, p. 1709-1714.
Steven, B., Leveille, R., Pollard, W., and Whyte, L.G., 2006, Microbial ecology and biodiversity in permafrost: Extremophiles, v. 10, p. 259-267.
Steven, B., Briggs, G., McKay, C.P., Pollard, W., Greer, C.W., and Whyte, L.G., 2007a, Characterization of the microbial diversity in a permafrost sample from the Canadian high Arctic using culture-dependent and culture-independent methods: FEMS Microbial Ecology, v. 59, p. 513-523.
Steven, B., Neiderberger, T.D., Bottos, E.M., Dyen, M.R., and Whyte, L.G., 2007b, Development of a sensitive radiorespiration method for detecting microbial activity at subzero temperatures: Journal of Microbiological Methods, v. 71, p. 275-280.
Steven, B., Chen, M.Q., Greer, C.W., Whyte, L.G., and Neiderberger, T.D., 2008, Tumebacillus permanentifrigoris gen. nov., sp. nov., an aerboic, spore-forming bacterium isolated from Canadian high Arctic permafrost: International Journal of Systematic and Evolutionary Microbiology, v. 58, p. 1497-1501.
Sukumaran, P.V., 2005, Magnetotactic bacteria, magnetofossils and the antiquity of life: Current Science, v. 88, p. 879-885.
Summons, R.E., Janhke, L.L., Hope, J.M., and Logan, G.A., 1999, 2-methyl hopanoids as biomarkers for cyanobacterial oxygenic photosynthesis: Nature, v. 400, p. 554-557.
Summons, R.E., Albrecht, P., McDonald, G., and Moldowan, J.M., 2008, Molecular biosignatures: Space Science Reviews, v. 135, p. 133-159.
Suttle, C.A., 2005, Viruses in the sea: Nature, v. 437, p. 356-361.
Suttle, C.A., 2007, Marine viruses ─ major players in the global ecosystem: Nature Reviews in Microbiology, v. 5, p. 801-812.
Talbot, H.M., Summons, R.E., Jahnke, L.L., Cockell, C.S., Rohmer, M., and Farrimond, P., 2008, Cyanobacterial bacteriohopanepolyol signatures from cultures and natural environmental settings: Organic Geochemistry, v. 39, p. 232-263.
Taran, Y.A., Kliger, G.A., and Sevastianov, V.S., 2007, Carbon isotope effects in the open-system Fischer Tropsch synthesis: Geochemica et Cosmochemica Acta, v. 71, p. 4474-4487.
Theimens, M.H., 2006, History and applications of mass-independent isotope effects: Annual Reviews in Earth and Planetary Science, v. 34, p. 217-262.
Thomas-Keprta, K.L., Clemett, S.J., Bazylinski, D.A., Kirshvink, J.L., McKay, D.S., Wentworth, S.J., Vali, H., Gibson, E.K., and Romanek, C.S., 2002, Magnetofossils from ancient Mars: A robust biosignature in the Martian meteorite ALH84001: Applied and Environmental Microbiology, v. 68, p. 3663-3672.
Wanger, G., Onsott, T.C., and Southam, G., 2008, Stars of the terrestrial deep sub-surface: A novel ‘star-shaped’ bacterial morphotype from a South African platinum mine: Geobiology, v. 6, p. 325-330.
Ward, J.A., Slater, G.F., Moser, D.P., Lin, L.H., Lacrampe-Couloume, G., Bonin, A.S., Davidson, M., Hall, J.A., Mislowack, B., Bellamy, R.E.S., Onstott, T.C., and Sherwood Lollar, B., 2004, Microbial hydrocarbon gases in the Witwatersrand Basin, South Africa: Implications for the deep biosphere: Geochimica et Cosmochimica Acta, v. 68, p. 3239-3250.
Whitman, W.B., 1998, Procaryotes: The unseen majority: Proceedings of the National Academy of Sciences, v. 95, p. 6578-6583.