Series
Economic Geology Models 2.
Melt Inclusions of Native Silver and Native Bismuth: A Re-examination of Possible Mechanisms for Metal Enrichment in Five-Element Deposits
Dan MarshallDepartment of Earth Sciences
Simon Fraser University
Burnaby, BC Canada, V5A 1S6
marshall@sfu.ca
Submitted May, 2008. Accepted as revised November, 2008
SUMMARY
This paper presents preliminary observations on veinlets and trails of native bismuth and silver melt inclusion that cross-cut silicate and carbonate vein fill and alteration minerals in the five-element veins at Cobalt, Canada. The low melting temperature of bismuth (271°C) is consistent with the current estimates of vein formation at Cobalt, and melt textures are displayed in native bismuth inclusions and trails. Native silver displays identical textures and these are also interpreted to have formed from a melt. However, native silver melts above 950°C, which is in direct conflict with current estimates of silver deposition within the Cobalt camp. In light of the similarities in textures, existing temperature evidence, the lack of experimental studies in the Co–As–Ag ternary, and recent advances in the study of melt inclusions in sulfide deposits, the native silver textures are also interpreted to have formed at temperatures as low at 350°C.A primary three-phase fluid inclusion assemblage contained within growth-zoned quartz crystals in the granophyric phase of the Nipissing diabase was chosen as representative of the highest temperature fluids responsible for ore deposition at Cobalt. This fluid inclusion assemblage displays microthermometric behaviour similar to the hypersaline fluid inclusions previously determined as the transporting medium for the silver mineralization at Cobalt and are consistent with depositional temperatures of about 350°C. These temperatures, although sufficient to produce melt inclusions of native bismuth, are insufficient to melt silver. Petrography and solid inclusion textures are consistent with metallic silver melts, indicating that Ag–Sb–Hg ternary or more complex silver-bearing systems containing H2O, H2S and salts may have eutectics at temperatures below 350°C. This is interpreted as a potential mechanism for silver mobilization and enrichment, and has potential applications to other types of vein mineralization.
SOMMAIRE
L'article suivant décrit des observations préliminaires effectuées sur des filon-nets minéralisés et des trainées d'inclusions vitreuses de bismuth et d'argent natifs recoupant les carbonates et les silicates de remplissage des filons ainsi que les minéraux d'altération des filons à cinq éléments à Cobalt, Canada. La basse température de fusion du bismuth (271°C) concorde avec les inter-prétations actuelles sur la formation des filons à Cobalt, ainsi qu'avec les textures de fusion visibles tant dans le bismuth natif que dans les trainées. L'argent natif montre aussi des textures identiques, lesquelles sont aussi comprises comme des indices de fusion. Cela dit, la température de fusion de l'argent natif dépasse 950°C, ce qui contredit carrément les estimations courantes concernant les dépôts d'argent dans le camp minier de Cobalt. Considérant la similarité des textures, les indications de température, le manque d'études expérimentales sur le comportement du sysème ternaire Co–As–Ag, et de percées récentes dans le cadre d'études d'inclusions fluides dans des gisements de sulfures, nous estimons qu'il est raisonnable de penser que les textures d'argent natifs ont pu se former à des températures aussi basse que 350°C.Un assemblage primaire à trois phases d'inclusions fluides au sein de cristaux de quartz à zones de croissance de la phase granophyrique de la diabase de Nipissing a été retenu comme indicateur de la température maximale des fluides à l'origine de la formation du gisement de Cobalt. Cet assemblage d'inclusions fluides affiche un comportement microthermométrique semblable à celui des inclusions hypersaline dont on a montré précédemment qu'il avait été le transporteur de la minéralisation d'ar-gent de Cobalt et qui correspond à des températures de formation d'à peu près 350°C. Bien que ces températures soient suffisantes pour expliquer la formation d'inclusions vitreuses de bismuth natif, elles ne peuvent expliquer la fusion de l'argent. Les données pétrographiques et les textures des inclusions solides justifient l'hypothèse de fusion de l'argent métal, et permet-tent de croire que le système ternaire Ag–Sb–Hg ou des systèmes argentifères plus complexes renfermant de H2O, H2S et des sels peuvent avoir des points eutectiques à des températures inférieures à 350°C. Nous pensons qu'il pourrait s'agir de mécanismes de mobilisation et d'enrichissement de l'argent, et qu'il pourrait être considérer dans d'autres cas de minéralisation filonienne.
INTRODUCTION
1 The silver-arsenide veins at Cobalt, Ontario and the Silver Islet equivalents near Thunder Bay are typical of the five-element (Co–Ni–As–Ag–Bi) vein assemblage reviewed by Kissin (1993). This deposit type has historically produced a great deal of the world’s silver. The most notable occurrences worldwide of the five-element-type vein system are Kongsberg in Norway (Bugge 1978; Johnsen 1986), Jáchymov in the Czech Republic (Ondrus et al. 2003), the Great Bear deposits in Canada (Ruzicka and Thorpe 1996), and the Bou Azzer deposits in Morocco (En-Naciri et al. 1997; Essaraj et al. 2005). Other deposits display some characteristics of the five-element vein type, but require further study, such as parts of the Idaho Cobalt Belt; Wickenberg, Arizona; the Black Hawk district in New Mexico; and Sabinal and Batopilas in Chihuahua, Mexico (Kissin 1993). Although most five-element deposits display similar geological characteristics such as Co–Ni–Fe arsenide and sulfarsenide minerals occurring with native silver and bismuth in a gangue of carbonate (± silicates), the individual deposits may also display distinct differences, such as host lithology, age, tectonic setting and igneous affinity.
2 The Cobalt deposits have produced approximately 12.6 billion grams (445 million ounces) of silver since their discovery in 1903. Native silver and (to a lesser extent) native bismuth generally occur with cobalt-arsenides, sulfarsenides and sulfosalts in near-vertical carbonate (± silicate) veins cutting Huronian sedimentary rocks of the Gowganda Formation, Archean greenstones, and the Paleoproterozoic Nipissing diabase (Fig. 1). All major deposits have been found within a few hundred metres of the unconformity between the Archean and Huronian rocks, in general proximity to the Nipissing diabase and volcanogenic sulfide mounds within the Archean metavolcanic and metasedimentary rocks (Jambor 1971a; Petruk 1971a). Marshall et al. (1993) have shown that chloride complexes are the dominant ligand responsible for silver transport, with silver vein formation occurring over the temperature range 300 to 350°C and pressures constrained between 60 and 136 MPa (600 and 1360 bars).
3 Melt and fluid inclusions have become an increasingly important tool in determining the partitioning of metals between melts and fluids, and consequently in determining the genesis of a number of ore deposit types, including porphyry copper deposits (Kamenetsky et al. 1999; Halter and Heinrich 2006) and metamorphosed gold deposits (Tomkins and Mavro-genes 2002). Melt inclusions texturally and genetically resemble fluid inclusions (Bodnar and Student 2006) and display similar petrographic and para-genetic details; these details can be utilized in the study of a number of ore deposit types.
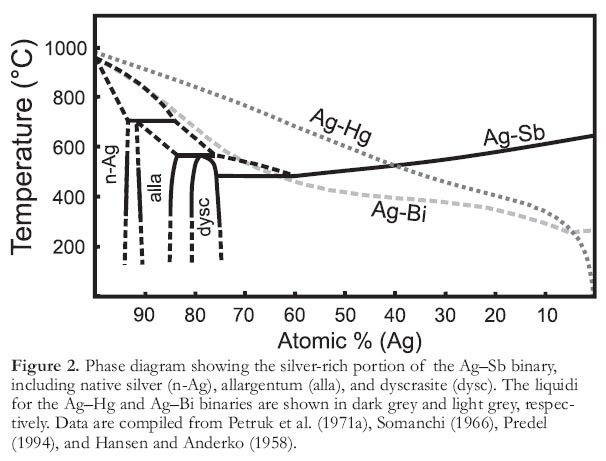
4 Based on melt inclusion textures from sulfides and gold in other deposits (Tomkins and Mavrogenes 2002; Wykes and Mavrogenes 2005; Tomkins et al. 2006) a hypothesis for native Bi and Ag solid inclusions is presented for the silver veins at Cobalt, Ontario. Both metals are reported in the veins at Cobalt (Petruk 1971b; Andrews et al. 1986; Marshall et al. 1993). The respective melting temperatures are 271.3 and 961.8°C, with the eutectic in the Bi–Ag binary system (Fig. 2) at 262°C (Hansen and Anderko 1958). The lack of experimental work describing phase relations and eutectics in these or more complex ternary systems, most notably Co–As–Ag and Fe–As–S–Bi, preclude a discussion of eutectic melts to describe these textures. However, the presence of low melting point metals such as mercury and bismuth, combined with typical melt textures such as skeletal crystal growth and solid inclusions, indicate that melt inclusions are a viable mechanism for mobilization and trapping native Ag and Bi in typical five-element veins.
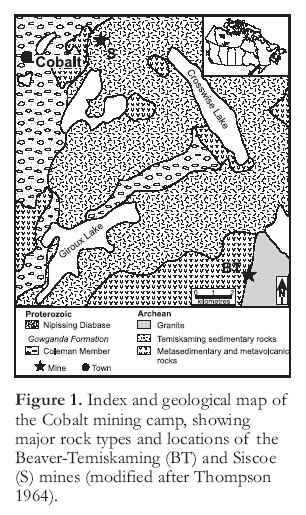
Figure 1. Index and geological map of the Cobalt mining camp, showing major rock types and locations of the Beaver-Temiskaming (BT) and Siscoe (S) mines (modified after Thompson 1964).REGIONAL GEOLOGY
5 The Cobalt mining camp occurs in the northeastern part of the Southern geological province, close to the boundary of the Superior and Grenville provinces. Archean metavolcanic and metasedimentary rocks are unconformably overlain by Proterozoic rocks of the Huronian Supergroup. The Archean and Proterozoic rocks have been intruded by the tholeiitic Nipissing diabase sills. The diabase sills are generally ~300 m thick and comprise a range of rock types from fine-grained border facies through coarse-grained amphibole-bearing diabase to late stage granophyric diabase. These rocks are unconformably overlain by Silurian and Ordovician limestones followed by Pleistocene and Recent sediments.
6 The oldest rocks in the Cobalt camp are Archean metavolcanic rocks and associated interflow sedimentary rocks (Jambor 1971a). The former are composed dominantly of intermediate to mafic flows containing some pyroclastic units and felsic volcanic rocks, and minor interflow sedimentary rocks (including chert and sulfide units; Nicols 1988), iron formation, and schist (Smyk 1987). Unconformably overlying the volcanic rocks are ‘Timiskaming-type’ lithic and feldspathic arenites, wackes and conglomerates (Jambor 1971a). These rocks were intruded by Archean granites followed by mafic, ultramafic and lamprophyric dikes and sills, and were subsequently metamorphosed to greenschist facies and folded isoclinally during the Kenoran Orogeny.
Figure 2. Phase diagram showing the silver-rich portion of the Ag–Sb binary, including native silver (n-Ag), allargentum (alla), and dyscrasite (dysc). The liquidi for the Ag–Hg and Ag–Bi binaries are shown in dark grey and light grey, respectively. Data are compiled from Petruk et al. (1971a), Somanchi (1966), Predel (1994), and Hansen and Anderko (1958).
Display large image of Figure 2
7 Proterozoic sedimentary rocks of the Gowganda Formation unconformably overlie the Archean rocks in the Cobalt area. A basal conglomerate above the Archean rocks is assigned to the Coleman Member of the Gowganda Formation; the Coleman Member predominantly consists of conglomerate, sandstone and laminated siltstone (Scammell 1984; Mustard 1985; Rain-bird 1985), and has a maximum thickness of 180 m. It is conformably overlain by the Firstbrook Member, consisting of laminated siltstone having a maximum thickness of 610 m (Jambor 1971a). The Gowganda Formation is conformably overlain by the Lorrain Formation, which has a maximum observed thickness in excess of 300 m and is cut by an erosional surface; this thickness includes only the lower arkosic unit and not the overlying quartz arenite (Jambor 1971a).
8 The Nipissing diabase sills intrude the Archean metavolcanic and Proterozoic sedimentary rocks. The sills are well differentiated and have a bulk composition of olivine tholeiite (Hriskevich 1968; Jambor 1971b); some gabbroic dikes are also present (Andrews et al. 1986). In general, the sills are shallowly dipping and form basin and dome structures (Petruk 1971a), although locally the diabase has been shown to follow pre-existing faults (Thompson 1967). Mineralogical and textural zoning within the lowest zone of the Nipissing diabase is manifested by a thin chilled margin 5-10 mm thick (Hriskevich 1968; Jambor 1971b), grading upward into the lower quartz diabase, which has a thickness of 15 to 30 m. Quartz diabase grades upward into hypersthene diabase, which forms up to two-thirds of the sill thickness. The hypersthene diabase grades upward into diabase with variable texture and grain size, and is locally aplitic, granophyric or pegmatitic. The variable-textured diabase is gradational into an upper quartz diabase that is generally not as thick as the lower quartz diabase. The contact with the intruded country rocks is marked by an upper chilled margin up to 10 mm thick.
9 A major southeast-trending fault system is manifested by the Montreal River, Cross Lake, and Quinze Dam faults (Wilson 1986). These faults can be traced up to hundreds of kilometres and represent one of the three major fault systems in the Cobalt area. Precambrian movement along these faults is difficult to prove, but the intrusion of a post-Nipissing diabase dike parallel to the Cross Lake fault may indicate zones of weakness in Precambrian times (Thompson 1967). The second fault set trends northeast, and the largest of these, the Cobalt Lake fault, offsets the Nipissing dia-base prior to silver mineralization (Thompson 1967). These faults and the southeast-trending system are generally veined with carbonate and silicate minerals and exhibit no apparent control over the occurrence of the silver veins, as most are barren (Jambor 1971a). The third set of faults, trending east-southeast, are generally smaller, sub-vertical normal faults that show displacements of up to 7.5 m, and locally host silver veins (Wilson 1986).
10 Vertical ‘ore veins’ and ‘sub-horizontal’ or flat veins are the two main types of veins in the Cobalt area (Andrews et al. 1986), and both occupy either shear zones or zones of dilation. Field relationships indicate that the flat veins are coeval with or later than the ore veins (Andrews et al. 1986). The ore veins are dominated by carbonate minerals, and silver-arsenides and sulfarsenides of cobalt, nickel and iron. The veins are typically zoned, and exhibit distinctive Ni–As, Ni–Co–As, Co–As, Co–Fe–As, Fe–As and Co–As assemblages; silver grades are highest in the Ni-bearing assemblages (Petruk 1971b). Ore veins vary in width from a few millimetres to more than 30 cm, and average less than 5 cm (Jambor 1971a); in places they are over 300 m long and 100 m deep, but are not necessarily ore grade over the entire length and depth. Flat veins are generally less abundant, thinner (up to 15 cm), and are dominated by silicate minerals (quartz, potassic feldspar, epidote, and axinite) and carbonate. Intersections of flat and ore veins are commonly preferred sites for ore deposition (Andrews et al. 1986; Petruk 1971a). Hydrothermal wall-rock alteration associated with the veins (Appleyard 1980) is minor and extends only up to several centimetres into the host rocks.
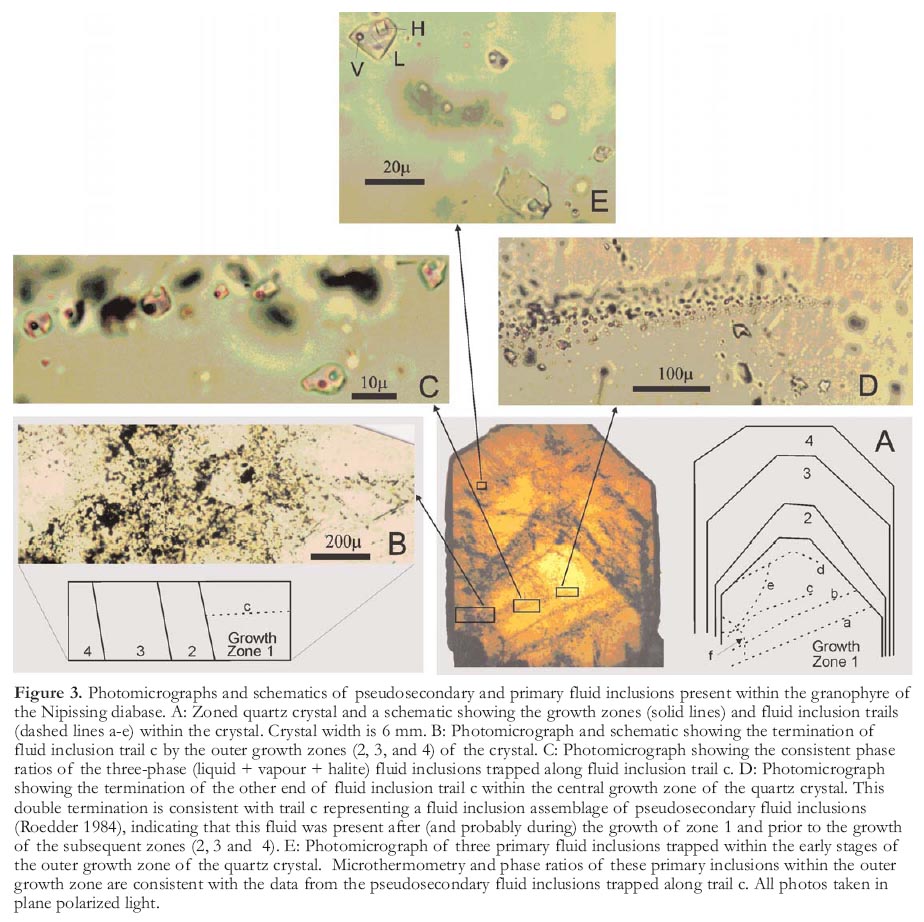
Figure 3. Photomicrographs and schematics of pseudosecondary and primary fluid inclusions present within the granophyre of the Nipissing diabase. A: Zoned quartz crystal and a schematic showing the growth zones (solid lines) and fluid inclusion trails (dashed lines a-e) within the crystal. Crystal width is 6 mm. B: Photomicrograph and schematic showing the termination of fluid inclusion trail c by the outer growth zones (2, 3, and 4) of the crystal. C: Photomicrograph showing the consistent phase ratios of the three-phase (liquid + vapour + halite) fluid inclusions trapped along fluid inclusion trail c. D: Photomicrograph showing the termination of the other end of fluid inclusion trail c within the central growth zone of the quartz crystal. This double termination is consistent with trail c representing a fluid inclusion assemblage of pseudosecondary fluid inclusions (Roedder 1984), indicating that this fluid was present after (and probably during) the growth of zone 1 and prior to the growth of the subsequent zones (2, 3 and 4). E: Photomicrograph of three primary fluid inclusions trapped within the early stages of the outer growth zone of the quartz crystal. Microthermometry and phase ratios of these primary inclusions within the outer growth zone are consistent with the data from the pseudosecondary fluid inclusions trapped along trail c. All photos taken in plane polarized light.
Display large image of Figure 3
11 A K/Ar age of 2525 ± 72 Ma (Jambor 1971a) from the quartz monzonite pluton that intrudes Archean metavolcanic rocks southeast of Cobalt provides an upper age constraint on the volcanic rocks. The Gowganda Formation has yielded a whole rock Rb/Sr isochron age of 2288 ± 87 Ma (Fairbairn et al. 1969). The silver veins cut the diabase and are therefore synchronous or younger. The U–Pb analyses of primary baddeleyite from the Nipissing diabase and vein-related secondary rutile have yielded ages of 2217.5 ± 1.6 and 2217.0 ± 6 Ma, respectively (Andrews et al. 1986) indicating contemporaneous diabase emplacement and silver mineralization.
FLUID INCLUSIONS
12 A variety of techniques, including microthermometry, cryogenic energy dispersive spectral (EDS) analyses, Raman microprobe studies and secondary ion mass spectrometry (SIMS) have been used to characterize the fluid inclusions in the Cobalt mining camp (Scott and O’Connor 1971; Ker-rich et al. 1986; Marshall et al. 1993). These studies reveal at least two distinct assemblages of fluid inclusions hosted within ore veins. A highly saline, three-phase assemblage consisting of a halite cube, a brine, and a vapour bubble at room temperature is the predominant fluid inclusion type. Fluid inclusion compositions are CaCl2 rich with a CaCl2: NaCl: KCl of approximately 3:2:1 and an overall salinity of approximately 35 wt% NaCl equivalent. These fluid inclusions have been shown petrographically to preand post-date deposition of silver within the Cobalt camp, and have also been shown to be the most probable transporting medium for the silver (Marshall et al. 1993). A second, less-abundant fluid inclusion assemblage is represented at room temperature by two-phase fluid inclusions. The two phases are an aqueous brine and a vapour bubble in varying proportions, with salinities ranging from almost pure water to 25 wt% NaCl equivalent. This fluid inclusion assemblage has also been shown to be contemporaneous with silver mineralization (Marshall et al. 1993). No appreciable concentrations of gases such as CO2, H2S, CH4 or N2 have been detected in either of the two fluid inclusion assemblages.
Table 1. Microthermometric data from primary and pseudosecondary three-phase (L+V+H) fluid inclusions hosted within zoned quartz of the granophyric phase of the Nipissing diabase at Peterson Lake, Ontario.

Display large image of Table 1
Display large image of Figure 4
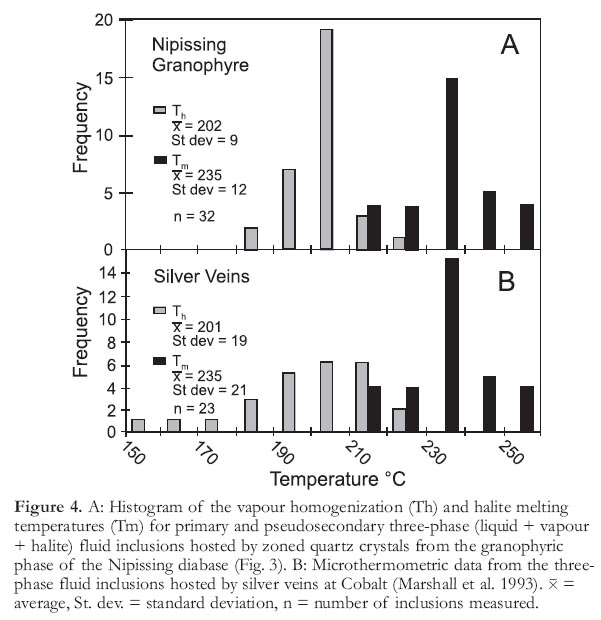
13 Primary and pseudo-secondary three-phase fluid inclusions (Fig. 3) in growth-zoned quartz crystals from a granophyric part of the Nipissing dia-base are represented at room temperature by vapour, brine, and a halite cube. The proportions of the three phases are similar to those reported by Marshall et al. (1993) for the primary three-phase fluid inclusions and the probable silver transporting fluid. Microthermometric measurements of the granophyre-hosted fluid inclusions (Table 1) and the three-phase fluid inclusions of Marshall et al. (1993) also show very similar patterns (Fig. 4).
SOLID INCLUSIONS
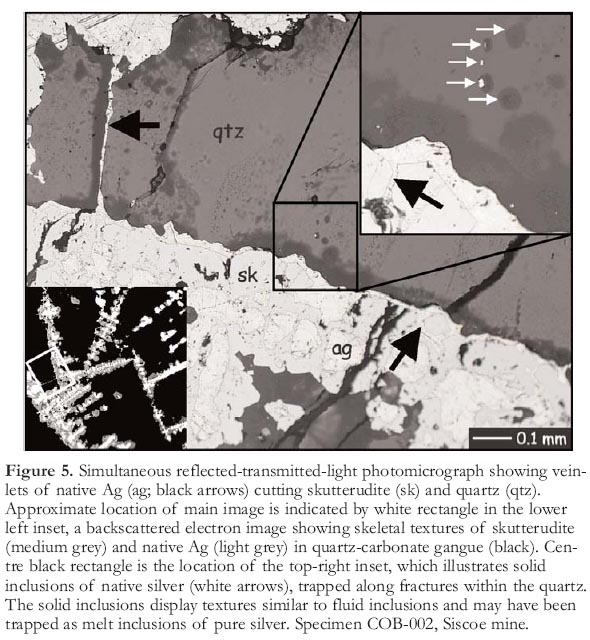
14 Native silver is the most common ore of silver within the Cobalt camp. It most commonly occurs as cores of rosettes or cruciform Ni–Co–Fe-sulfarsenides and as veinlets cutting the sulfarsenides. The veinlets range in width from micrometres to centimetres. The smaller veinlets of native silver are often accompanied by or lead into trails of native silver inclusions that can be seen in outcrop and polished thick sections (Fig. 5). These textures are suggestive of precipitation from a melt and are similar to higher temperature melt inclusions observed in the Challenger gold deposit (Tomkins and Mavrogenes 2002). The solid inclusions of native silver within the polished thick sections were examined petrographically and with SEM and microprobe. The native silver contains variable amounts of Sb and Hg, ranging up to 8.4 and 8.1 mass percent, respectively (this study; Petruk et al. 1971b). No other sulfides or sulfarsenides have been observed within the trails of solid silver inclusions. However, rare solid inclusions trapped along healed fractures have apophyses of saline brine associated with them, indicating that a brine component may also be part of the silver-bearing (metallic) melt. Additionally, the reaction of metallic melts with water would lower melting temperatures in the Ag–Sb–Hg system, and the presence of chloride brines, H2S, HS- and other aqueous sulfide species may similarly lower melting temperatures (Wykes and Mavrogenes 2005).
Figure 5. Simultaneous reflected-transmitted-light photomicrograph showing vein-lets of native Ag (ag; black arrows) cutting skutterudite (sk) and quartz (qtz). Approximate location of main image is indicated by white rectangle in the lower left inset, a backscattered electron image showing skeletal textures of skutterudite (medium grey) and native Ag (light grey) in quartz-carbonate gangue (black). Centre black rectangle is the location of the top-right inset, which illustrates solid inclusions of native silver (white arrows), trapped along fractures within the quartz. The solid inclusions display textures similar to fluid inclusions and may have been trapped as melt inclusions of pure silver. Specimen COB-002, Siscoe mine.
Display large image of Figure 5
Display large image of Figure 6
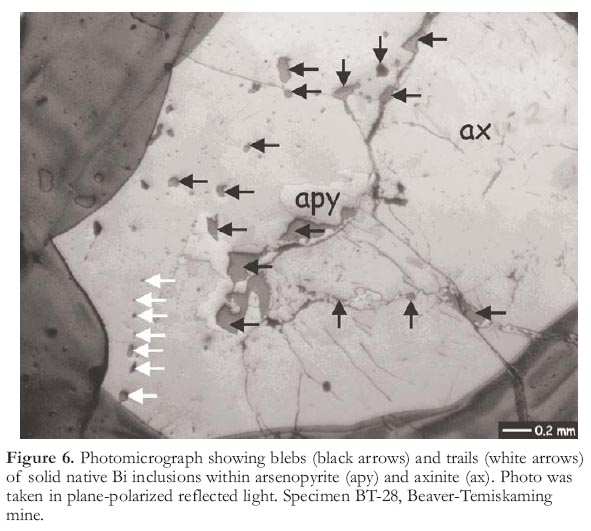
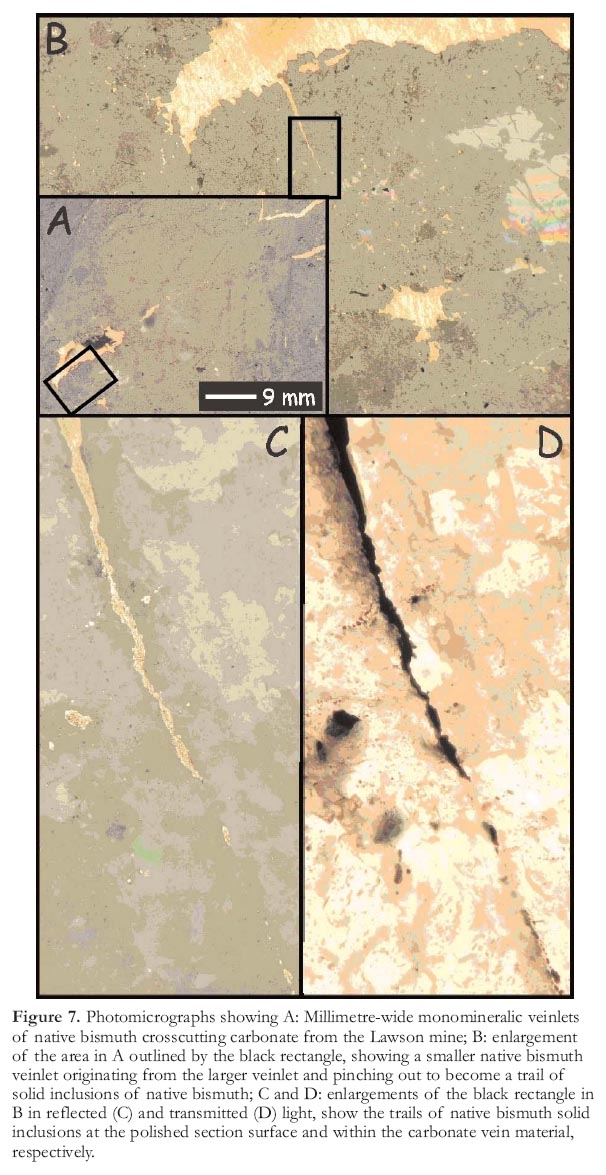
15 Solid inclusions of native bismuth, although less common, are also present in the Cobalt area, as veinlets and irregular masses (Petruk 1971a) and as trails of solid inclusions (Figs. 6, 7). Bismuth is also reported within the silver veins, where it "interchanges intermittently with native silver" (Petruk 1971a). The veinlets and trails of solid bismuth inclusions range in width up to hundreds of microns and crosscut carbonates, silicates, and sulfarsenides within ore veins. Trails of solid bismuth inclusions were analyzed by electron microprobe and were shown to consist of pure bismuth. Bismuth would be molten at the pressure and temperature conditions of vein formation, and thus, the solid inclusions of native bismuth are interpreted as melt inclusions. Furthermore, melting in the Ag–Bi binary occurs at approximately 260°C (Hansen and Anderko 1958), which is easily within the range of temperatures derived for silver deposition at Cobalt (Kerrich et al. 1986; Marshall et al. 1993). As both the silver and bismuth inclusions display similar textures, it is probable that some of the silver at Cobalt was also mobilized and deposited as melt inclusions.
DISCUSSION and CONCLUSIONS
16 Fluid inclusion studies on zoned quartz crystals from the granophyric phase of the Nipissing diabase have been used to determine the pressures, temperatures, and compositions of the fluids associated with the Nipissing granophyres. The results are consistent with previous fluid inclusion studies from the study area and reveal that the saline brines previously reported from the region and deemed partially responsible for the transport and deposition of silver in the Cobalt region were in equilibrium with the later stages of the Nipissing intrusions. Thus, they had at the very least equilibrated with the diabase or possibly exsolved from the diabase during cooling.
Figure 7. Photomicrographs showing A: Millimetre-wide monomineralic veinlets of native bismuth crosscutting carbonate from the Lawson mine; B: enlargement of the area in A outlined by the black rectangle, showing a smaller native bismuth veinlet originating from the larger veinlet and pinching out to become a trail of solid inclusions of native bismuth; C and D: enlargements of the black rectangle in B in reflected (C) and transmitted (D) light, show the trails of native bismuth solid inclusions at the polished section surface and within the carbonate vein material, respectively.
Display large image of Figure 7
17 Saline brines have been found within crystallized metallic melt inclusions and the presence of such brines coexisting or dissolved within the metal-rich melts would not only depress the melting temperatures of the metals and metal mixtures, but would also aid in the healing of the melt-inclusion trails as the solubility of gangue minerals such as quartz and calcite is higher in saline brines than in molten metals. Hydrogen fugacity has been shown to have a pronounced effect on the solubilities and melting temperatures of chalcopyrite (Mavro-genes and Bodnar 1994). Higher hydrogen fugacities, brines, and aqueous sulfur species present during the deposition of the silver veins at Cobalt may also have lowered the melting temperatures of native metals and their alloys.
18 Petruk’s (1971b) comprehensive study reported that silver increases in grade from low in Fe–As and Ni–As assemblages, to medium in Co–As and Co–Fe–As vein assemblages, to high in Ni–Co–As and Co–As assemblages. This may be related to eutectic temperatures in the various chemical systems and is significant to the study of primary silver deposition or remobilization in other types of silver deposits.
19 Previous evidence for the temperatures and pressures of vein formation at Cobalt are in excess of those required to melt native bismuth and some of the textures in native bismuth are therefore interpreted to be crystallized metallic melt inclusions. Similar textures in native silver from the same deposits indicate that some silver may have been mobilized as a melt as well. The presence of native metal melt inclusions does not suggest that all Ag and Bi were deposited from metal-rich melts; however, mobilization of native metals may be responsible for some of the observed textures in the veins and for some remobilization and concentration of Ag and Bi within the five-element (Ni–Co–As–Ag–Bi) veins at Cobalt and similar occurrences worldwide.
ACKNOWLEDGEMENTS
The help of numerous individuals from the Department of Earth Sciences at Carleton University, especially P. Jones, G. Skippen and D.H. Watkinson is gratefully acknowledged. Early discussions with Larry Diamond as well as suggestions and communications from Ian Jonasson and John Mavrogenes improved an earlier version of this manuscript. Reviews by David Lentz and two anonymous reviewers also contributed to this paper. An NSERC discovery grant to DM is also gratefully acknowledged.REFERENCES
Andrews, A.J., Owsiacki, L., Kerrich, R., and Strong, D. F., 1986, The silver deposits at Cobalt and Gowganda, Ontario. I: Geology, petrography, and whole-rock geochemistry: Canadian Journal of Earth Sciences, v. 23, p. 1480-1506.
Appleyard, E.C., 1980, Host-rock alteration at the Silverfields mine, Cobalt, Ontario – final report: Ontario Geological Survey, Geoscience Research Grant No.70, 1979-1980, 83 p.
Bodnar, R.J., and Student, J.J., 2006, Melt inclusions in plutonic rocks: Petrography and microthermometry, in Webster, J.D., ed., Melt Inclusions in Plu-tonic Rocks: Mineralogical Association of Canada, Short Course 36, p. 1-25.
Bugge, J.A.W., 1978, Kongsberg-Bamble complex, in Bowie, S.H.O., Kvalheim, A., Haslan, M.W., eds., Mineral Deposits of Europe: Northwest Europe: Institution of Mining and Metallurgy and Mineralogical Society, London, v. 1, p. 213-217.
En-Naciri, A., Barbanson, L., and Touret, J.-C., 1997, Brine inclusions from the Co-As(Au) Bou Azzer district, Anti-Atlas Mountains, Morocco: Economic Geology, v. 92, p. 360-367.
Essaraj, S., Boiron, M.C., Cathelineau, M., Banks, D.A., and Benharref, M., 2005, Penetration of surface-evaporated brines into the Proterozoic basement and deposition of Co and Ag at Bou Azzer (Morocco): Evidence from fluid inclusions: Journal of African Earth Sciences, v. 41, p. 25-39.
Fairbairn, H.W., Hurley, P.M., Card, K.D., and Knight, C.J., 1969, Correlation of radiometric ages of Nipissing diabase and Huronian metasediments with Proterozoic orogenic events in Ontario: Canadian Journal of Earth Sciences, v. 6, p. 489-497.
Halter, W., and Heinrich, C., 2006, Magmatic processes and volatile phase generation in porphyry-type environments: A laser ablation-ICP-MS study of silicate and sulfide melt inclusions, in Webster, J.D., ed., Melt Inclusions in Plutonic Rocks: Mineralogical Association of Canada, Short Course 36, p. 151-164.
Hansen, M., and Anderko, K., 1958, Constitution of Binary Alloys: McGraw Hill, New York, 1305 p.
Hriskevich, M.E., 1968, Petrology of the Nipissing diabase sill of the Cobalt area, Ontario, Canada: Geological Society of America Bulletin, v. 79, p. 1387-1404.
Jambor, J.L., 1971a, General geology of the Cobalt Area: Canadian Mineralogist, v. 11, p. 12-33.
Jambor, J.L., 1971b, The Nipissing diabase: Canadian Mineralogist, v. 11, p. 34-75.
Johnsen, O., 1986, Famous mineral localities: the Kongsberg silver mines, Norway: Mineralogical Record, v. 17, p. 19-36.
Kamenetsky, V.S., Wolfe, R.C., Eggins, S.M., Mernagh, T.P., and Bastrakov, E., 1999, Volatile exsolution at the Dinkidi Cu-Au porphyry deposit, Philippines: A melt-inclusion record of the initial ore-forming process: Geology, v. 27, p. 691-694.
Kerrich, R., Strong, D.F., Andrews, A.J., and Owsiacki, L., 1986, The silver deposits at Cobalt and Gowganda, Ontario. III: Hydrothermal regimes and source reservoirs – evidence from H, O, C and Sr isotopes and fluid inclusions: Canadian Journal of Earth Sciences, v. 23, p. 1519-1550.
Kissin, S.A., 1993, Five-element (Ni-Co-As-Ag-Bi) veins, in Sheahan, P.A., and Cherry, M.E., eds., Ore Deposit Models II: Geoscience Canada Reprint Series, 6, p. 87-98.
Marshall, D.D., Diamond, L.W., and Skip-pen, G.B., 1993, Silver transport and deposition at Cobalt, Ontario, Canada: Fluid inclusion evidence: Economic Geology, v. 88, p. 837-854.
Mavrogenes, J.A., and Bodnar, R.J., 1994, Hydrogen movement into and out of fluid inclusions in quartz: Experimental evidence and geologic implications: Geochimica et Cosmochimica. Acta, v. 58, p. 141-148.
Mustard, P.S., 1985, Sedimentology of the lower Gowganda Formation Coleman Member (early Proterozoic) at Cobalt, Ontario, Canada: Unpublished MSc thesis, Carleton University, Ottawa, Ontario, 143 p.
Nicols, R.S., 1988, Archean geology and silver mineralization controls at Cobalt, Ontario: Canadian Institute of Mining and Metallurgy Bulletin., v. 81, no. 910, p. 40-48.
Ondrus, P., Veselovsky, F., Gabasova, A., Hlousek, J., Srein, V., Vavrin, I., Skala, R., Sejkora, J., and Drabek, M., 2003, Primary minerals of the Jachymov ore district: Journal of the Czech Geological Society, v. 48, p. 19-147.
Petruk, W., 1971a, General characteristics of the deposits, in Berry, L.G., ed., The Silver-Arsenide Deposits of the Cobalt-Gowganda Region, Ontario: Canadian Mineralogist, v. 11, p. 76-107.
Petruk, W., 1971b, Mineralogical characteristics of the deposits and textures in the ore minerals, in Berry, L.G., ed., The Silver-Arsenide Deposits of the Cobalt-Gowganda Region, Ontario: Canadian Mineralogist, v. 11, p. 108-139.
Petruk, W., Harris, D.C., Cabri, L.J., and Stewart, J.M., 1971a, Characteristics of the silver-antimony minerals: Canadian Mineralogist, v. 11, p. 187-195.
Petruk, W., Jambor, J., and Boyle, R.W., 1971b, History of the Cobalt and Gowganda area: Canadian Mineralo-gist, v. 11, p. 1-7.
Predel, B., 1994, Landolt-Bornstein, Group IV Physical Chemistry - Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys: Springer-Verlag, 354 p.
Rainbird, R.H., 1985, Sedimentology and geochemistry of the Firstbrook Member of the Gowganda Formation in the eastern Cobalt Basin, Ontario: Unpublished M.Sc. thesis, Carleton University, Ottawa, Ontario, 87 p.
Roedder, E., 1984, Fluid Inclusions, Reviews in Mineralogy: Mineralogical Society of America, v. 12, 644 p.
Ruzicka, V., and Thorpe, R.I., 1996, Arsenide silver-cobalt veins, in Eck-strand, O.R., Sinclair, W.D., and Thorpe, R.I., eds., Geology of Canadian Mineral Deposit Types: Geological Survey of Canada, Geology of Canada, No. 8, p. 287-306.
Scammel, R. J., 1984, The geology of the Precambrian strata east of Cobalt, Ontario: Unpublished B.Sc. thesis, Carleton University, Ottawa, Ontario, 71 p.
Scott, S.D., and O’Connor, T.P., 1971, Fluid inclusions in vein quartz, Silverfields Mine: Canadian Mineralogist, v. 11, p. 263-271.
Smyk, M.C., 1987, Geology of Archean interflow sedimentary rocks and their relationship to Ag-Bi-Co-Ni-As veins, Cobalt area, Ontario: Unpublished M.Sc. thesis, Carleton University, Ottawa, Ontario, 87 p.
Somanchi, S., 1966, Subsolidus phase relations in the system Ag-Sb: Canadian Journal of Earth Sciences, v. 3, p. 211-222.
Tomkins, A.G., and Mavrogenes, J.A., 2002, Mobilization of gold as a poly-metallic melt during pelite anatexis at the Challenger Deposit, South Australia: A metamorphosed Archean gold deposit: Economic Geology, v. 97, p. 1249-1271.
Tomkins, A.G., Frost, B.R., and Pattison, D.R.M., 2006, Arsenopyrite melting during metamorphism of sulfide ore deposits: Canadian Mineralogist, v. 44, p. 1045-1062.
Thompson, R., 1964, Cobalt silver area, northern sheet, Timiskaming district: Ontario Department of Mines, Map 2050.
Thompson, R., 1967, Cobalt and district: Canadian Institute of Mining and Metallurgy, Centennial Field Excursion, Northwestern Québec–Northern Ontario, p. 136-143.
Wilson, B.S., 1986, A sulphur isotope and structural study of the silver vein host rocks at Cobalt, Ontario: Unpublished M.Sc. thesis, Carleton University, Ottawa, Ontario, 156 p.
Wykes, J.L., and Mavrogenes, J.A., 2005, Hydrous sulfide melting: Experimental evidence for the solubility of H2O in sulfide melts: Economic Geology, v. 100, p. 157-164.