Series
International Year of Planet Earth 2.
Earth and Health — Building a Safer Canadian Environment
Pat E. RasmussenHealth Canada Healthy Environments and Consumer Safety Branch 50 Colombine Driveway Ottawa, ON, Canada, K1A 0K9
pat_rasmussen@hc-sc.gc.ca
H.D. Gardner
Department of Earth Sciences University of Ottawa, Marion Hall, Room 121 Ottawa, ON, Canada, K1N 6N5
Submitted June, 2008. Accepted as revised October, 2008
SUMMARY
This article reviews the current state of Earth and Health research in Canada, in commemoration of International Year of Planet Earth. Canadian geoscientists play a pivotal role in identifying sources and pathways of exposure to geochemical substances that may be beneficial or detrimental to human health. Application of geoscience techniques and expertise contributes to understanding the sources and pathways of natural versus anthropogenic contaminants; bioaccumulation and biomagnification of contaminants in remote settings; and regional variability in background levels of elements and their compounds in the environment. Internationally, the relationship of earth sciences to human health has become so important that it has given rise to a new field of study termed ‘Medical Geology’. Within Canada, there are vivid examples of the impact of geological materials and processes on human health. Mineralogists, geochemists and toxicologists have started collaborating to quantify proportions of metals in soil and dirt, household dust, and playground substrates that are soluble and potentially available for absorption in the body. Other current issues include the new radon guidelines; distribution patterns of mercury in Canadian Shield lakes and reservoirs; geological sources of arsenic and fluoride in groundwater; selenium in Cretaceous sediments; exposures to airborne particles, some of which have natural sources such as windblown dust, forest-fire debris, and volcanic ash; and urban geochemistry with focus on childhood exposures to lead. Geoscience research into sources and pathways of metals and other geochemical hazards improves the accuracy and reliability of human health risk assessments that underlie risk management policies and decision-making.SOMMAIRE
À l'occasion de l'Année internationale de la planète Terre, le présent article passe en revue l'état de la recherche en sciences de la Terre et en sciences de la santé au Canada. Les géoscientifiques canadiens ont un rôle charnière dans la détermination des sources et des voies d'exposition aux substances géochimiques pouvant être bénéfiques ou nocives pour la santé. L'application des techniques et des connaissances géoscientifiques aident à déterminer les sources et les voies d'exposition naturelles et anthropogéniques, les mécanismes de bioaccumulation et de bioamplification sur des sites éloignés ainsi que la variabilité régionale des concentrations de fond des éléments et de leurs composés dans l'environnement. Sur le plan international, la relation entre les sciences de la Terre et les sciences de la santé est d'une importance telle qu'un nouveau champ de recherche est né, la "géologie médicale". Au Canada, on peut voir des exemples frappants de l'impact qu'ont des matériaux et des mécanismes géologiques sur la santé humaine. Les minéralogistes, les géochimistes et les toxicologistes ont commencé à mettre en commun leurs efforts dans le but de quantifier les proportions de métaux solubles présents dans le sol et la saleté, les poussières domestiques et les sols de terrains de jeux, pouvant être absorbés à travers l'épiderme. Parmi d'autres questions d'importance, il y a les nouvelles lignes directrices sur le radon; les profils de distribution du mercure dans les lacs et les réservoirs du Bouclier canadien; les sources d'arsenic et de fluorure dans les eaux souterraines; le sélénium dans les sédiments crétacés; les types d'exposition aux particules en suspension dans l'air, dont certaines sont d'origine naturelle comme les poussières éoliennes, les cendres de feux de forêt ou des éruptions volcaniques, et; la géochimie urbaine, avec un accent sur l'exposition au plomb des enfants. La recherche géoscientifique sur les sources les voies d'exposition des métaux et autres risques géochimiques permet d'améliorer la précision et la fiabilité des évaluations du risque pour la santé humaine qui sous-tendent les politiques et la prise de décision en matière de gestion de risque.INTRODUCTION
1 The United Nations General Assembly proclaimed 2008 as the ‘International Year of Planet Earth (IYPE)’. In recognition that a main theme of IYPE is ‘Earth and Health – Building a Safer Environment’ [http://www.yearofplanetearth.org], this article provides a snapshot of current research in the area of geochemical hazards, which is a very important Earth and Health issue for Canadians.
2 Geochemical hazards represent only one facet of the emerging field of ‘Medical Geology’, which was recently defined by Berger (2003) as the study of "public health impacts of geological materials and processes." Davies et al. (2005) broadened the definition to "the study of health problems related to ‘place’ ". Although the scope of the present article is limited to inorganic contaminants, with particular focus on metals and their compounds, recent books (e.g. Nolan et al. 2001; Skinner and Berger 2003; Selinus et al. 2005) show that geochemical hazard research covers many more subjects, including waterborne pathogens, occupational exposures to asbestos, organic compounds such as polycyclic aromatic hydrocarbons, and spatial modelling, to name but a few. The study of geochemical hazards is at the core of Medical Geology, and is an increasingly important part of environmental health research in Canada.
3 This examination of current Canadian research into inorganic geochemical hazards reveals a complex field that links environmental toxicology, geology, and human health risk assessment. Canadians are exposed to metals and their compounds in a variety of ways, primarily by the inhalation of metals in the workplace or polluted neighbourhoods, through childhood ingestion of dust and soil containing lead and other metals, drinking well water, and through the consumption of certain foodstuffs in which metals tend to bioconcentrate (such as shellfish). Overexposure to metals and their compounds have been associated with a wide range of adverse health effects (see Merian et al. 2004), including neurological effects (e.g. lead and methylmercury), birth defects (methylmercury), damage to the renal system (e.g. cadmium chloride, uranium, elemental mercury, and mercuric chloride), cancer (e.g. arsenic, hexavalent chromium), and allergic reactions (e.g. beryllium, nickel, and chromium). Most recently, metals in airborne particles have been linked to cardio-respiratory effects (see Burnett et al. 2000) because of the role of transition metals (e.g. zinc, vanadium, copper) in oxidation processes that affect lung function. Airborne hazards also include radon and airborne particulate matter emitted from a wide range of natural and industrial sources. Elements that are essential to health, such as selenium and fluorine, are also the subject of current Canadian research as these elements vary widely in the natural environment depending on the geological setting and biogeochemical cycling, and can become toxic if consumed in high doses (Fig.1).
Figure 1. A specific combination of conditions is required for selenium to enter the food chain in significant concentrations. These conditions are: the presence of a geological source of selenium (e.g. Cretaceous shales), the presence of a selenium accumulator species (Astragalus racemosus), and alkaline conditions which keep selenium in its soluble forms available for uptake. Map adapted from Outridge et al. (1999).

Display large image of Figure 1
4 The purpose of investigating sources and pathways of human exposure to metals and other geochemical hazards is to improve the accuracy and reliability of human health risk assessments that underlie public policy and decision-making. Geoscientists contribute to this effort by identifying and quantifying: a) the sources of hazardous elements in the surrounding environment (natural and anthropogenic); b) the chemical forms in which the elements occur (that govern their bioavailability); and c) the exposure pathway (i.e. inhalation, ingestion, or dermal exposure). Many specific examples of geochemical hazards research have been published recently (e.g. Reimann and Garrett 2005; Reeder et al. 2006; Edmunds and Smedley 2005; Fuge 2005).
ADVANCES IN DISTINGUISHING NATURAL AND ANTHROPOGENIC SOURCES
5 Distinguishing natural and anthropogenic sources of metals in the environment is arguably the most difficult challenge faced by Canadian geochemists working on environmental health issues (Rasmussen 1996). The last ten years has witnessed significant advances in this aspect of metals research (see Reimann and Garrett 2005). In Canada, this improvement has been driven largely by the necessity to clearly define and quantify ‘background concentrations’ in site-specific risk assessments for contaminated sites. Federal contaminated sites are located on lands owned or leased by the federal government, or on non-federal lands where the federal government has accepted full responsibility for the contamination. At present there are 18 300 sites listed on the Federal Contaminated Sites Inventory (Environment Canada 2008). This inventory, which is maintained by the Treasury Board Secretariat, includes harbours, historic landfills, factories, mines, military bases, airports, laboratories, and lighthouse stations.
6 Interestingly, if concentrations at a given site are found to be less than or equal to the local naturally occurring background concentrations of those elements, the site may not be designated ‘contaminated’ even if the human health guidelines are exceeded. The term ‘background concentration’ is defined variously by the individual provinces and territories that have jurisdiction over their contaminated sites, but generally refers to the concentration of a substance at a given site exclusive of any contribution from local anthropogenic point sources of contamination. Guidance provided by the Yukon protocol (Yukon Department of Environment 2007), for example, indicates that, "a site is not a contaminated site if it does not contain any contaminant with a concentration greater than or equal to the local background concentration of that contaminant in the soil, groundwater, or drinking water."
7 Case studies of arsenic contamination from past mining activities in Canada provide excellent examples of the relevance of geoscience in sitespecific risk assessments. Richardson (2002) noted that the natural soil-borne levels of arsenic in Yellowknife average approximately 150 mg/kg, with natural levels occasionally exceeding 1500 mg/kg. Thus, the local background is one to two orders of magnitude higher than the Canadian Council of Ministers of the Environment (CCME) residential soil quality guideline for arsenic, which is 12 mg/kg (CCME 1997). The federal government has designated the site of the former Giant Mine in Yellowknife as a Federal Contaminated Site because contamination of the environment by the mine operations significantly exceeds background concentrations. In this case, geoscience played a crucial role in distinguishing arsenic contamination associated with mining activities from naturally elevated concentrations in the surrounding environment.
DRINKING WATER: A CLEAR EXAMPLE OF THE ROLE OF GEOSCIENCE IN POPULATION HEALTH
8 Some of the most striking examples of the impact of geology on human health in Canada are cases where trace elements (both essential and harmful) are leached into groundwater from geological materials. In addition to the known contaminated sites located across Canada, geoscientists and public health authorities continue to discover areas of naturally elevated trace element concentrations throughout the country. Arsenic is a good example. Generally, arsenic levels in Canadian groundwater supplies are less than 5 μg/L, which is half the Canadian guideline for safe drinking water (Health Canada 2006). However, concentrations above this guideline occur naturally in groundwater in small areas of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Newfoundland and Labrador, and Nova Scotia. Where arsenic concentrations exceed safe standards for drinking water, a number of certified drinking water treatment devices can be employed to reduce arsenic concentrations to safe limits for residential use (Health Canada 2006).
9 Nova Scotia, which is characterized by bedrock locally enriched in trace elements and a long history of gold mining, presents geoscientists with unique challenges in addressing both natural and anthropogenic sources of arsenic. Naturally elevated arsenic concentrations were found in water supplies in parts of Nova Scotia as early as the 1970s, notably in Halifax and Guysborough counties. In Waverley, on the outskirts of Halifax, arsenic contamination of groundwater, derived from tailings of gold mines that operated between the 1860s and 1930s, overprints the bedrock-derived arsenic concentrations (Grantham and Jones 1977). Recent multidisciplinary studies have focused on assessing and reducing risks associated with historical gold mines in Nova Scotia (Natural Resources Canada 2007a). A special issue of the journal Geochemistry: Exploration, Environment, Analysis is devoted to the legacy of mining in Nova Scotia and will be published in 2009.
10 Depending on the local geological setting, rural well water is a potential route for residential exposure to other naturally occurring elements such as fluorine and uranium. The Atlas of Canada shows that groundwater is the principal source of drinking water for about eight million people in Canada, or 26% of the population, and that approximately two-thirds, or five million, of these users live in rural areas [http://atlas.nrcan.gc.ca]. Young children are especially sensitive to uranium from this route of exposure because of the developmental immaturity of their kidneys and other organs, in combination with the large volume of water they consume in proportion to their body mass.
11 The benefit of fluoride in the reduction of dental caries is well established, but in well waters, fluoride concentrations that exceed the drinking water standard must be identified to prevent potential overexposure. Recently, the Ontario Geological Survey (Nicks et al. 2007) reported elevated fluoride concentrations exceeding drinking water guidelines (1.5 mg/L) in 40% of the 534 groundwater sampling stations in southwestern Ontario. Many people living in this area rely on groundwater as their main potable water supply. Other geogenic elements of secondary concern were uranium and arsenic, which, in rare cases, approached or exceeded the provincial standards (Nicks et al. 2007).
12 Municipal water drawn from deep aquifers in southwestern Ontario is commonly naturally fluoridated. Municipal water supplies in Perth, Waterloo, Oxford, and Wellington counties in Ontario are in this group. The phenomenon of elevated fluoride concentrations throughout the area is associated predominately with calcium–magnesium-bicarbonate Paleozoic bedrock of the Dundee Formation and Detroit River Group, and with overburden wells in Wisconsinan glacial deposits (Nicks et al. 2007). Fluoride surveys conducted in other Canadian provinces and territories in the 1980s revealed that some communities in the Maritime Provinces, Québec, Saskatchewan and Alberta had concentrations as high as 2.52–4.35 mg/L, exceeding the 1.5 mg/L guideline recommended by Health Canada (Droste 1987). In particular, fluoride is associated with sedimentary rocks that underlie large parts of the eastern provinces. In the community of Maria, on the Gaspe Peninsula of Québec, elevated fluoride levels (5–28 mg/L) in well water affected 15–20% of the population (Boyle and Chagnon 1995). More recently, in Manitoba, Leybourne et al. (2008) followed up on earlier studies that reported elevated fluoride concentrations in domestic wells associated with the Lake St. Martin meteorite impact structure near Gypsumville, where 20% of samples exceeded 1.5 mg/L. Fluoride levels in groundwater influenced by associated evaporites and redbeds are also elevated in this area, and have a mean concentration of 1.68 mg/L (Leybourne et al. 2008). Once identified, these groundwater sources can be effectively treated by municipalities or residents by using reverse osmosis or distillation techniques to reduce fluoride concentrations in drinking water to acceptable levels.
BIOACCUMULATION AND BIOMAGNIFICATION IN REMOTE AREAS
13 Although advances have been made in distinguishing natural and anthropogenic sources at contaminated sites, source apportionment in remote areas remains a difficult challenge. In the example of selenium bioaccumulation in Astragalus racemosus (common name: cream milkvetch, a member of the pea family), source apportionment is unambiguous because of the presence of a known bedrock source (Cretaceous shale; Fig. 1) in combination with the specific geochemical conditions that allow uptake by a selenium accumulator species. However, in most settings there are contributions of metals from a variety of natural and anthropogenic sources, and the processes of bioaccumulation and biomagnification can obscure the origins of the metals, making source apportionment very complex. The concept of biomagnification, or biological magnification, which is the increase in concentration of a substance along the food chain, became famous in the 1960s following studies of the behaviour of the pesticide DDT. In the case of metals such as mercury and cadmium, which typically occur in μg/kg (ppb) concentrations in geological materials and bioaccumulate to the mg/kg (ppm) range in the food chain, the relative influence of geological compared to industrial sources can be very difficult to ascertain in remote areas.
14 The bioaccumulation of cadmium in the kidneys of caribou is a good example. Caribou have been an important part of many First Nations diets for thousands of years. Even today, caribou and moose meat are the main sources of protein, iron, zinc, copper and magnesium in Dene and Métis communities along the Mackenzie River (Van Oostdam et al. 2003). Concern associated with the consumption of caribou is restricted to the kidneys, as cadmium primarily accumulates in this organ (Gamberg and Scheuhammer 1994). The cadmium and other trace elements in caribou come from the lichen on which they graze. The fungi and algae that compose lichen absorb cadmium from locally derived windblown dust and soil particles and from longer range atmospheric transport of air pollutants, forest fires and volcanic eruptions. Given the vast grazing areas covered by the migrating caribou herds, pinpointing the precise origins of the cadmium that ends up in caribou kidney, and quantifying with certainty the relative contributions of natural and anthropogenic sources, is a daunting task.
15 Mercury presents dramatic examples of biomagnification, even in ecosystems that do not have direct inputs from local industrial sources. In contrast to the previous example of bioaccumulation of cadmium in caribou, which occurs within an individual (caribou are herbivores), biomagnification of mercury occurs across trophic (food chain) levels. Rasmussen et al. (1998a) described large variations in mercury concentrations amongst lakes in the vicinity of Huntsville, Ontario, and showed how biomagnification processes led to extraordinarily high mercury concentrations (> 2.0 mg/kg) in the smallmouth bass of some lakes, with contrastingly low mercury concentrations (< 0.5 mg/kg) in smallmouth bass of the same age and length in neighbouring lakes. Analysis of bottom sediments from 25 lakes around Huntsville showed an order-of-magnitude variability in sediment mercury concentrations (from < 5 μg/kg to > 450 μg/kg) among the Huntsville lakes (Rasmussen 1993). This variability is comparable to the variability in mercury concentrations reported for lakes across Canada (Rasmussen et al. 1998b). After mercury concentrations in the bottom sediments are normalized for organic carbon content, the spatial pattern of sediment mercury (in the μg/kg or ppb range) closely matches the spatial distribution of fish mercury (in the mg/kg or ppm range), with the highest fish mercury concentrations exceeding 2.5 mg/kg, over five times the consumption guideline (Rasmussen 1993). Analysis of ragweed pollen (Ambrosia sp.) and carbon-14 dating provided a chronological framework, and revealed that local geology exerts a strong control on the spatial distribution of mercury in deep precolonial sediments as well as in the modern environment in the Huntsville area of the Precambrian Shield.
16 In 2001, a multi-disciplinary team of research scientists, including meteorologists, chemists, biologists, geologists, and limnologists, formed the Collaborative Mercury Research Network [COMERN www.unites.uqam.ca/comern/], a fiveyear research program aimed at better understanding the processes leading to mercury exchange and accumulation in large Canadian ecosystems. Kejimkujik National Park, Nova Scotia was one of the COMERN study sites. As a result of biomagnification, loons in the Kejimkujik ecosystem have the highest blood levels of mercury of any breeding loon population tested in North America (Burgess et al. 2005). Mercury has an affinity for organic compounds, and Kejimkujik has many lakes that are high in humic acids (giving the water a brown colour) and correspondingly high mercury levels. Similar to the Huntsville case study by Rasmussen (1993, 1998a), Rencz et al. (2003) concluded that mercury distribution in the Kejimkujik study area is not explained solely by atmospheric sources, and turned their attention to local watershed sources, quantifying mercury concentrations in all environmental compartments, including bedrock, soil, and vegetation. They concluded that geology is important, not only as one of the sources of mercury, but also as a driver for the type of environment in Kejimkujik Park. This includes the growth of coniferous forest and the occurrence of brown lakes that have a low buffering capacity, low pH, and long flushing times. These lake conditions ultimately contribute to elevated mercury concentrations in the resident yellow perch fish population, which is the principal food supply for loons at the top of the food chain in the Kejimkujik ecosystem (Rencz et al. 2003).
17 The complexity of the biogeochemical cycling of mercury is captured in the cross-disciplinary Mineralogical Association of Canada Short Course "Mercury Sources, Measurements, Cycles and Effects" (Parsons and Percival 2005). One chapter describes individual ‘custom-designed’ approaches that were developed for monitoring gaseous elemental mercury emissions from naturally mercuriferous bedrock and surficial materials across Canada (Rasmussen et al. 2005). Gaseous elemental mercury is released to the atmosphere from terrestrial landscapes during physical and chemical weathering of soils and minerals at the air-surface interface. Point sources are represented by small carbonaceous shale outcrops and quarries near Thunder Bay, Ontario; area sources are represented by a regional scale mercuriferous shale outcrop in southeastern Yukon; and line sources are represented by a mineralized fault zone in southeastern Ontario (Rasmussen et al. 2005). Other chapters describe advances in analytical measurement technologies, approaches to distinguishing natural and anthropogenic sources of mercury, current modelling approaches, and management practices to reduce the impact of mercury and its various compounds on environmental and human health.
BIOAVAILABILITY AND BIOACCESSIBILITY
18 Canadian mineralogists, geochemists and toxicologists are actively collaborating on research aimed at investigating the properties of metal compounds in soil and dirt, household dust, and playground substrates, to understand their potential availability for absorption in the body (Metals in the Human Environment Strategic Network (MITHE-SN) 2008). Knowledge of the biological availability and toxic action of metal compounds in dust and soil particles is considered essential for identifying risks to human health that arise from ingesting or inhaling dust and soil particles at contaminated sites (Richardson et al. 2006). Currently, there is a lack of quantitative information on the composition of particles in typical indoor dust. A new Canadian working group entitled BioAccessibility Research Canada (BARC 2007) is currently developing and comparing a wide range of chemical extraction tests that mimic the human digestive system, with the aim of improving the accuracy of exposure assessments. Research in metal ‘bioaccessibility’ is used to estimate the soluble (and potentially available) quantity of particle-bound metals inside the human body. This research is driven by the realization that measurements of the total metal content of dust or soil provide little relevant information on the fate of metals in the human body, after the particle-bound metal enters the digestive tract or the lung environment.
19 Regulatory agencies are currently examining the potential role of bioavailability and bioaccessibility testing when conducting risk assessments on contaminated sites (International Society of Exposure Analysis (ISEA) 2007). The United States Environmental Protection Agency (US-EPA; 2007a) defines bioavailability as "the fraction of an ingested dose that crosses the gastrointestinal epithelium and becomes available for distribution to internal target tissues and organs." It is now widely recognized that the most common, and most precautionary, default assumption of 100% bioavailability is unrealistic in many situations. Some regulators are beginning to consider a correction for bioavailability provided that the testing meets rigorous criteria involving well-designed and appropriate animal models (ISEA 2007).
20 Although chemical extraction procedures to estimate bioaccessibility are useful in research applications, they are unlikely to be accepted in regulatory applications unless or until they are carefully validated by an appropriate animal model. Recently, the US-EPA accepted a physiologically-based chemical extraction method developed by Drexler and Brattin (2007) for site specific human health-risk assessments of lead in contaminated soils (US-EPA 2007b). Acceptance of this method, which is a simulated stomach acid extraction, occurred only because Drexler and Brattin (2007) were able to demonstrate that their in vitro measurements of bioaccessible lead in soils correlate well with in vivo measurements of relative bioavailability, therefore meeting the US-EPA acceptance criteria.
21 Demonstrating a strong correlation between bioaccessibility and true bioavailability is difficult. A glance at the Drexler and Brattin (2007) reference list shows that almost a decade elapsed from the development of their approach for estimating bioaccessible lead to acceptance by the regulators in 2007. Researchers in the Canadian BARC initiative, aware that regulatory uptake of bioaccessibility research for other metals and metalloids will take a long time, are taking a collaborative approach by performing carefully designed inter-laboratory comparisons (BARC 2007).
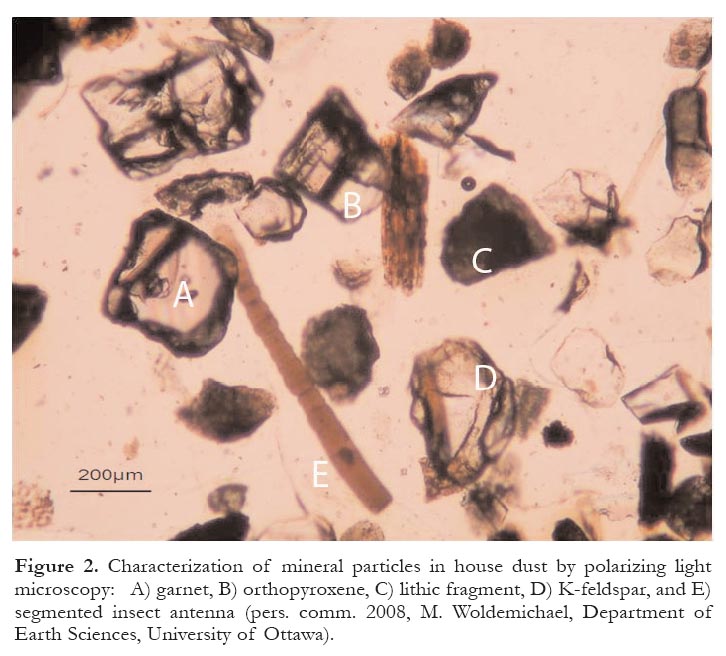
22 Metal speciation, one of the key factors controlling the bioaccessibility of particle-bound metals, is being explored using innovative applications of synchrotron x-ray spectroscopic techniques, in solid samples of Canadian indoor dust (Rasmussen et al. 2008) and mine tailings (Corriveau et al. in press). Currently there is a lack of quantitative information on the composition of particles in typical indoor dust. Polarizing light microscopy (Fig. 2) and scanning electron microscopy (Fig. 3) are being used to address this data gap by characterizing the mineralogy of household dust sampled from homes across Canada.
23 Particle size is another key factor influencing bioaccessibility. Small particles (e.g. < 100 μm) adhere better to a child’s hand and are thus more likely to be ingested than large particles. Ingested small particles (and associated metals) are often more bioaccessible than larger particles (Rasmussen et al. 2008). Therefore, sieving dust and soil to appropriate size fractions (Fig. 4) is a critical aspect of exposure measurement studies. With respect to the inhalation pathway, aerodynamic particle size is a key parameter determining the fate of the inhaled particle in the lung environment.
THE INHALATION PATHWAY OF EXPOSURE
24 The relationship between solubility and potential toxicity of inhaled particles in the lung environment is completely different from that in the gastrointestinal environment. Solubility of a particle in the lung environment influences its clearance time, or 'biopersistence'. In their overview of physiochemical properties of particles that are relevant explain that the longer a particle persists unaltered in the lung, causing stress to cells and tissues, the greater the extent of any adverse effect. Advanced mineralogical and biogeochemical analysis techniques are required to explore the toxicological relevance of shape, surface reactivity, chemical composition of particles (including metal content and organic content), and interactions between biogenic and inorganic components at the particle surface. Sahai (2007) describes the challenges, both intellectual and institutional, that mineralogists face as they investigate the role and behaviour of minerals, amorphous solids, nanocrystals, and nanoclusters in the human body.
Figure 2. Characterization of mineral particles in house dust by polarizing light microscopy: A) garnet, B) orthopyroxene, C) lithic fragment, D) K-feldspar, and E) segmented insect antenna (pers. comm. 2008, M. Woldemichael, Department of Earth Sciences, University of Ottawa).
Display large image of Figure 2

Display large image of Figure 3
25 Fueling this area of research is the ever-increasing demand for knowledge about the effect of airborne particles on health – and fine particles (< 2.5 μm) are of particular concern because they are able to penetrate to the deepest parts of the lungs (Health Canada 2007b). Elevated concentrations of fine particulate matter are associated with many health problems, including asthma, bronchitis, shortness of breath, painful breathing, and premature death. Children tend to be more susceptible to the health risks of particulate matter because their respiratory systems are still developing; they breathe in more air and, consequently, more particulate matter per kilogram of body mass than adults. The elderly, whose immune systems are more likely to be weakened due to age or other health problems, are also more susceptible than the general adult population.
26 Outdoor and indoor air contains a variety of fine particles in the nm to μm size range that are derived from both natural and industrial sources. Important airborne biogenic particles include viruses, bacteria, fungi, spores, waxes, leaf and needle fragments, and algae. Apart from biogenic particles, other important natural sources of fine particulate matter include wind-blown dust and forestfire debris. Volcanic eruptions are of particular interest to geoscientists as a source of airborne ash and dust particles. Large, explosive volcanic eruptions can eject into the atmosphere enough dust to encircle the planet and cause climate change on a global scale. Canadians from British Columbia to Manitoba experienced ash fall from the 1980 eruption of Mount St. Helens in Washington State (Stasiuk et al. 2006). The 1992 eruption of Mount Spurr in Alaska deposited enough ash in the Yukon Territory to close the Alaska Highway for a few hours due to reduced visibility. Ash particles can be less than 0.001 mm in diameter (< 10 μm), which is the inhalable fraction of airborne dust. Compared to health effects of urban air pollution, however, the risks associated with inhalation of volcanic ash are limited, and the greatest impact is usually experienced by individuals with pre-existing pulmonary conditions like asthma or emphysema (Horwell and Baxter 2006).
27 Indoor air quality is increasingly an area of concern because Canadians spend the greatest proportion of their time indoors. The penetration of vehicle emissions and airborne particles from industrial sources can introduce outdoor contaminants, metals and organic compounds into the home. In addition, the home contains many indoor sources of metal and organic contaminants, including consumer products, carpets, furnishings, paints, building materials, cooking processes, cigarette smoke, crafts and hobbies (Rasmussen 2004a). Results of residential air monitoring studies in Ontario indicate that indoor elemental concentrations in Windsor (Rasmussen et al. 2007a) and in Ottawa (Rasmussen et al. 2006) are approximately three to five orders of magnitude lower than indoor occupational exposure limits. In general, these studies found that the lowest airborne elemental concentrations most often occur in the indoor home environment, compared to outdoor and personal exposure samples, and thus the greatest analytical challenge lies in accurately quantifying metals in indoor air samples. To accurately measure very low indoor air concentrations, great care must be taken to avoid or minimize sources of contamination in the field and in the laboratory (Rasmussen et al. 2006; 2007a).
THE NEW CANADIAN RADON GUIDELINE
28 Recently, Canadian epidemiologists estimated that residential radon is responsible for 10% of the human lung cancer burden (Krewski et al. 2005; Zielinski et al. 2006). In 2007, Health Canada responded by lowering the Canadian indoor exposure guideline for radon to 200 becquerels per cubic metre (Bq/m3; Canada Gazette 2007), which is four times more stringent than the former guideline of 800 Bq/m3 (a becquerel is defined as one nuclear decay per second). Therefore, homes that were previously considered to be safe might now be considered to have a radon problem requiring remediation. In homes where the average annual radon concentration in the normal occupancy area exceeds the guideline, remedial measures are recommended (Health Canada 2007a).
29 Bedrock sources of uranium, the most common source of radon gas, can be identified using airborne and ground-based gamma-ray spectrometers to measure radioactivity. The Geological Survey of Canada has been using these techniques to map radioactivity in Canada since 1967 (Natural Resources Canada 2007b). In human health applications, the goal is to use these maps to identify regions with high equivalent uranium concentrations in bedrock, sediment and soil, as elevated radon concentrations are likely to occur within some homes in such regions. An example of the value of such mapping comes from Nova Scotia, where measurements of radon in 719 homes in ten communities within the province correlated strongly with uranium concentrations inferred from spectrometric mapping (Jackson 1992).
30 Unfortunately, not all predictive geological mapping efforts are equally successful. The ability of the host material to transport radon (e.g. fractures in rock, interconnected pores in sediments) is one of many environmental variables controlling indoor radon concentrations (Chen et al. 2007a). Other factors include the characteristics of the building itself, such as cracks and holes in the foundation that allow radon to enter the home; the presence or absence of areas where radon gas can pool or accumulate in the home and the efficiency of home ventilation (Appleton 2005).
31 Within a given residential area, neighbouring homes can have very different radon concentrations; hence, the best information comes from direct measurements of radon inside individual homes (Chen et al. 2007b). A number of devices are available to make radon measurements, including charcoal canisters and alpha track detectors, which are placed in the home for a set collection period, then sent to certified laboratories for analysis. If the analysis indicates that radon levels within the home exceed 200 Bq/m3, then remedial action can be taken to minimize the risks associated with exposure (Canada Mortgage and Housing Corporation and Health Canada 2007).
EMERGING ISSUES: LOW-DOSE CHRONIC EXPOSURES, MIXTURES, AND RISK ASSESSMENT OF ESSENTIAL ELEMENTS
32 A key area of current environmental health research is the risk assessment of long-term (chronic), low-level exposure to potentially toxic elements, including metals such as lead, mercury, and cadmium, and metalloids such as arsenic. Risks associated with chronic exposures to lead are not limited to smelter towns, mine sites, and other industrially-impacted areas; they also exist in residential areas where some older homes still contain remnants of lead-based paint, lead pipes and solder. Small children, who are particularly vulnerable to lead’s neurotoxic effects, can become exposed because of their tendency to ingest dust and soil through normal hand-to-mouth activities. This year (2008) a new initiative called the "Lead Collaborative Consortium" was launched at McMaster University, to assess current knowledge gaps about lead and to outline strategies to minimize health impacts of lead in Canada.
33 In response to the growing demand for public health information on low-level exposures to contaminants, the last decade witnessed a burgeoning of urban geochemistry research. Recent literature reviews (Rasmussen 2004a; Filipelli et al. 2005) describe a wide range of studies on exposure of residents to metals (with lead receiving the greatest attention); advantages and disadvantages of various indoor sampling approaches; abatement technologies; mapping the distribution of metal concentrations in the urban environment; indoor/outdoor concentration ratios; and application of stable lead isotopic analysis for identification of sources and exposure pathways in the home environment (e.g. Gulson et al. 1995; Maddaloni et al. 1998; Manton et al. 2000). Centuries of using coal and lead paint in St. John’s, for example, has left a legacy of lead contamination in soils of the downtown area, as revealed by Professor Trevor Bell and his team at Memorial University (Bell 2008). In contrast, studies of urban residential ‘background’ environments that are not heavily impacted by industry, such as Ottawa, show that concentrations of lead and other metals such as copper, nickel and zinc may be many times higher in indoor dust than in exterior garden soil (Rasmussen et al. 2001), and that each metal may be more orally bioaccessible in indoor dust than in soil (Rasmussen 2004b; Rasmussen et al. 2008).
34 Emerging research into lowdose exposures includes efforts to assess toxicological effects of metal mixtures and their complex interactions in biological processes (Chapman 2008). The intricate interactions of mixtures may either enhance or mitigate toxicological effects that are observed for each individual metal. Characterizations of mixtures, rather than individual metals or metal compounds, are more representative of typical exposure scenarios in both natural and man-made environments, including soil and dust in and around contaminated sites and polluted urban neighbourhoods. Multi-element approaches are emphasized in metals research being conducted by MITHE-SN that is directed at addressing data gaps in environmental and human health risk assessments (MITHE-SN 2008).
35 Finally, the topic of deficiency must be considered, as no discussion of low-dose exposures to metals would be complete without the reminder that many trace elements are essential for human health (Olin 1998; Fordyce 2005; Fuge 2005). The dose-response behaviour of essential elements, such as selenium, zinc and copper, is completely different from that of nonessential elements, such as lead, cadmium, and mercury. The latter elements, which have no known benefits to human health, exhibit toxic effects at elevated levels of exposure or intake. In contrast, the essential elements have an optimum intake range. The doseresponse curve for essential elements is commonly depicted as ‘U-shaped’, whereby a decrease in intake below the optimum range leads to deficiency effects, and an increase in intake above the optimum range leads to toxic effects (see Olin 1998). The importance of refining human health risk assessments of essential elements was the subject of a recent workshop at University of Ottawa (McLaughlin Centre 2008).
CANADA'S ENVIRONMENTAL BURDEN OF DISEASE
36 Exposures to most environmental hazards are considered preventable, and as such, environmental health research is well-suited to policy intervention. Efforts are underway to quantify the economic and societal cost of health effects attributable to the environment, and while such calculations are associated with many uncertainties (see Proceedings of Workshop on Environmental Burden of Disease; McLaughlin Centre, 2007a), preliminary estimates indicate that the cost is significant. For example, the World Health Organization (WHO) estimated that 13% of the disease burden in Canada is attributable to the environment (WHO 2007). This year Boyd and Genuis (2008), a Canadian team, reported on their efforts to tease out the proportion of the burden of disease in Canada that can be attributed to environmental risk factors. They estimated that between $3.6 billion and $9.1 billion in healthcare costs occur in Canada each year because of respiratory disease, cardiovascular illness, cancer, and congenital afflictions associated with adverse environmental exposures. Their estimate included chemical, biological, and radiological hazards, and excluded risk factors that would be considered occupational. An important observation made by Boyd and Genuis (2008) is the current lack of nationally representative Canadian data that would be required to improve the accuracy and completeness of their calculations. They could not, for example, discuss the influence of environmental factors on neurodevelopmental disorders because of the current lack of data on the prevalence of this category of illness in Canada. This observation is relevant to environmental geochemists interested in metals because mercury and lead are high on the list of substances associated with neurodevelopmental problems. The authors strongly criticized the existing lack of reliable and representative Canadian biomonitoring data that would allow population-level exposures to environmental hazards to be determined.
37 On a positive note, within the last year several national-scale studies have been launched that will eventually (within five years) address the existing lack of Canadian biomonitoring and exposure data. The Canadian Health Measures Survey (CHMS) is being conducted by Statistics Canada [http://www.statcan.ca] in partnership with Health Canada and Public Health Agency Canada, and will provide national estimates of environmental chemicals in blood and urine, by age and gender. Sample collection will include 5000 Canadians aged 6 to 79 yrs and will take place over two years (2007–09) in 15 sites across Canada. The second cycle of the Canadian Health Measures Survey (2009–11) plans to include children less than 6 years of age.
38 Biomonitoring data are also being collected through the Maternal-Infant Research on Environmental Chemicals (MIREC). This five-year national study (2008–13) on maternal and neonatal exposure to environmental chemicals will recruit 2000 pregnant women from 10 Canadian cities to assess infant/mother exposure. This study is jointly funded by Health Canada [http://www.hc-sc.gc.ca], the Canadian Institute of Health Research, and the Ontario Ministry of the Environment.
39 On the subject of indoor environmental exposure, Health Canada launched the Canadian House Dust Study in 2007 to establish background levels for inorganic and organic chemicals in a typical urban Canadian single family dwelling [http://www.hcsc.gc.ca/ewh-semt/contaminants/dustpoussiere-eng.php]. This four-year national study involves the collection of vacuum dust samples from over 1000 randomly selected homes in cities across the country. After drying and sieving to appropriate size fractions (Fig. 4), the dust samples will be analyzed for a suite of chemicals to provide a robust estimate of average concentrations of metals and organic compounds representative of urban Canadian household dust (Rasmussen et al. 2007b). The Canadian House Dust Study will target the same list of substances as the two concurrent national biomonitoring studies – CHMS and MIREC – with the aim of providing mutually consistent information on body burdens and possible indoor routes of exposure in Canadian homes.
Figure 4. In residential surveys, house dust is collected using a vacuum sampler and is then air-dried and sieved to appropriate size fractions. Large particles such as coins and pet hair (top) are separated from the sieved fractions used for chemical analysis (less than 80 micron fraction, bottom right) and mineralogical characterization (80 to 150 micron fraction, bottom left). From Rasmussen et al. (2007b).

Display large image of Figure 4
40 Geospatial information – geological maps for earth scientists and epidemiological data for public health professionals – is an essential integrative tool that is fundamental to the activities of researchers from various disciplines. The recent US National Research Council (US-NRC) report on "Research Priorities for Earth Science and Public Health" called for increased data sharing between agencies to promote interdisciplinary research without compromising privacy (US-NRC 2007). A current example of this type of effort is the tri-national soil survey (Canada, USA and Mexico), known as the North American Soil Geochemical Landscapes Project (Natural Resources Canada 2008). The objective of this project is to provide representative soil geochemical data for North America. Using this information, geoscientists will contribute to epidemiological and exposure research by establishing regional background values, and identifying areas of enrichment related to anthropogenic and natural sources (McLaughlin Centre 2007b; Natural Resources Canada 2008).
41 Based on a review of the literature on soil contaminants as causative agents of disease, Hough (2007) concludes that, from an epidemiological viewpoint, there is a need for more individual-level studies to establish exposure to soil as a risk factor for disease. The primary approach used by geoscientists to study relationships between environment and human health is known as ‘aggregate-level’, and is directed toward broadly relating spatial soil characteristics to geographic incidence of disease (Hough 2007). Public health scientists and epidemiologists refer to such aggregate-level studies as hypothesis-forming, whereas individual-level approaches are considered to be the ‘gold-standard’ for establishing causative relationships (Hough 2007).
CONCLUSION
42 Numerous examples of public health issues associated with geological materials and processes underscore the relevance of geoscience to public health. Perhaps the most familiar application of geoscience is the characterization of contaminated sites (both anthropogenic and natural sources), where elevated concentrations of elements occur in drinking water, soil, food and air.
43 The contribution of geosciences in other areas of public health can be fully valued when integrated with research by toxicologists, epidemiologists and medical researchers involved in biomonitoring, exposure assessment and environmental health surveillance. This is where international initiatives like the North American Soil Geochemical Landscapes Project can provide valuable data that will assist in understanding the spatial distribution of various beneficial and/or toxic elements in soil. By integrating data collected from biomonitoring and environmental surveys, researchers will be able to identify subpopulations at risk, determine how risk can be mitigated, and establish safer residential environments through remediation and education.
44 With respect to the urban environment, by examining the composition and physical characteristics of airborne particulate matter, it may be possible to determine provenance (e.g. consumer products, geologic material, industrial pollutants). Novel applications of isotopic analysis and mineralogical techniques, such as synchrotron analysis, are being used to characterize the chemical composition of ingested and inhaled particles. This information is invaluable in understanding the process and pathways for the absorption of chemical constituents into the human body, and assists in the development of protocols for estimating bioavailability and bioaccessibility.
ACKNOWLEDGMENTS
The authors sincerely thank Jeanne Percival, Mike Parsons, Reg Wilson, Mike Wade and Michelle Nugent for their constructive reviews and many helpful suggestions for improving the manuscript, and Michael Woldemichael, André Lalonde and Peter Jones for providing the PLM and SEM images. PR gratefully acknowledges support from Health Canada’s Healthy Environments and Consumer Safety Branch, the University of Ottawa Earth Sciences Department, and the NSERC MITHE Strategic Network [www.mithe-sn.org].REFERENCES
Appleton, J.D., 2005, Radon in air and water, in Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U. and Smedley, P., eds., Essentials of Medical Geology, Impact of the Natural Environment on Public Health: Academic Press, p. 227-262.
Bell, Trevor, 2008, About lead in the environment: Department of Geography, Memorial University [http://www.mun.ca/geog/research/lead.php].
Berger, A., 2003, Linking health to geology, in Skinner, C.W. and Berger, A.R. eds , Geology and Health: Closing the Gap, Oxford University Press, p. 5-11.
BioAccessibility Research Canada (BARC), 2007, Final Proceedings of the BioAccessibility Research Canada (BARC) October 11-12, 2007: Strategic Research Workshop on Bioaccessibility/Bioavailability in Contaminated Site Assessment, Nov. 26, 2007 [http://www.cntc.ca].
Boyd, D.R., and Genuis, S.J., 2008, The environmental burden of disease in Canada: Respiratory Disease, Cardiovascular Disease, Cancer, and Congenital Affliction: Environmental Research, v. 106, p. 240-249.
Boyle, D.R., and Chagnon, M., 1995, An incidence of skeletal fluorosis associated with groundwaters of the Maritime Carboniferous basin, Gaspé Region, Quebec, Canada: Environmental Geochemistry and Health, v. 17, p. 5-12.
Burnett, R.T., Brook, J., Dann, T., Delocia, C., Philips, O., Cakmak, S., Vincent, R., Goldberg, M.S., and Krewski, D., 2000, Association between particulate and gas-phase components of urban air pollution and daily mortality in eight Canadian cities: Inhalation Toxicology, v. 12 (Supplement 4), p. 15-39.
Burgess, N.M., Evers, D.C., and Kaplan, J.D., 2005, Mercury and other contaminants in common loons breeding in Atlantic Canada: Ecotoxicology, v. 14, p. 241-252.
Canada Gazette, 2007, Regulations amending the Canada occupational health and safety regulations, in Canadian Gazette Part II, v.141, no.25, sors/dors/2007-271, p. 2445-2452. [http://canadagazette.gc.ca/partII/2007/20071212/pdf/g214125.pdf].
Canada Mortgage and Housing Corporation and Health Canada, 2007, Radon: A guide for Canadian homeowners, ISBN 0-662-25909-2 Cat. no. NH15180/1997E, p. 1-47.
Canadian Council of Ministers of the Environment (CCME), 1997, Canadian soil quality guidelines for the protection of environmental and human health: arsenic (inorganic), [http://documents.ccme.ca/download/en/257/], p. 1-7.
Chapman, P.M., 2008, Environmental risks of inorganic metals and metalloids: A continuing, evolving scientific odyssey: Human and Ecological Risk Assessment, v. 14, p. 5-40.
Chen, J., Falcomer, R., Bergman, L., Wierdsma, J., and Ly, L., 2007a, A pilot indoor and soil radon survey in Ottawa (abstract): Health Canada Science Forum, November 8-9, 2007 Book of Abstracts ISBN: H1-9/232007E. p. 2.07.
Chen, J., Jiang, H., Tracy, B.L, and Zielinski, J.M., 2007b, A preliminary radon map for Canada in the unit of health regions (abstract): Health Canada Science Forum, November 8-9, 2007, Book of Abstracts ISBN: H1-9/232007E. p. 2.06.
Corriveau, M.C., Jamieson, H.E., Parsons, M.B., and Hall, G.E.M., (in press), Mineralogical characterization of arsenic in gold mine tailings from three sites in Nova Scotia: Geochemistry: Exploration, Environment, Analysis.
Davies, B., Bowman, C., Davies, T., and Selinus O., 2005, Medical geology: Perspective and prospective, in Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U. and Smedley, P., eds., Essentials of Medical Geology, Impact of the Natural Environment on Public Health: Academic Press, p. 1-14.
Drexler J.W., and Brattin, W.J., 2007, An in vitro procedure for estimation of lead relative bioavailability: With validation: Human Ecological Risk Assessment, v. 13, p. 383-401.
Droste, R.L., 1987, Fluoridation in Canada as of December 31, 1986: Health and Welfare Canada, Ottawa, Ontario, (IWD-AR WQB-89-154).
Edmunds, M., and Smedley, P., 2005, Fluoride in natural waters, in Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U., and Smedley, P., eds., Essentials of Medical Geology, Impact of the Natural Environment on Public Health: Academic Press, p. 301-330.
Environment Canada, 2008, The federal contaminated sites action plan (FCSAP) overview and progress federal contaminated sites – National Workshop, Vancouver May 01, 2008, Powerpoint Presentation by Lisa Keller, P. Eng., Contaminated Sites Division, Environment Canada, [http://www.rpic-ibic.ca/en/activities/2008_FCS/paper_presentations/plenary.shtml]
Filipelli, G.M., Laidlaw, M.A.S., Latimer, J.C., and Raftis, R., 2005, Urban lead poisoning and medical geology: An unfinished story: GSA Today, v. 15, p. 4-11.
Fordyce, F., 2005, Selenium deficiency and toxicity in the environment, in Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U., and Smedley, P., eds., Essentials of Medical Geology, Impact of the Natural Environment on Public Health: Academic Press, p. 373-416.
Fubini, B., and Fenoglio, I., 2007, Toxic potential of mineral dusts: Elements, v. 3, p. 407-414.
Fuge, R., 2005, Soils and iodine deficiency, in Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U., and Smedley, P., eds., Essentials of Medical Geology, Impact of the Natural Environment on Public Health: Academic Press, p. 417-434.
Gamberg M., and Scheuhammer A. M., 1994, Cadmium in caribou and muskoxen from the Canadian Yukon and Northwest Territories: Science of The Total Environment, v. 143, p. 221-234.
Grantham, D.A., and Jones, J.F., 1977, Arsenic contamination of water wells in Nova Scotia: Journal of the American Water Works Association, v. 69, p. 653-657.
Gulson, B.L., Davis, J.J., and Bawden-Smith, J., 1995, Paint as a source of recontamination of houses in urban environments and its role in maintaining elevated blood leads in children: Science of the Total Environment, v. 164, p. 221-235
Health Canada, 2006, It’s your health: Arsenic in drinking water: [http://www.hc-sc.gc.ca/hl-vs/iyhvsv/environ/arsenic-eng.php], Catalogue No. H50-3/6-2004E-PDF, ISBN # 0-662-36233-0.
Health Canada, 2007a, It’s Your Health: Radon: [http://www.hc-sc.gc.ca/iyhvsv/environ/radon_eng.html].
Health Canada, 2007b, Priority substances list assessment report for respirable particulate matter: Environmental and Workplace Health, ISBN: 0-66228531-X, Cat. No.: En40-215/47E, [http://www.hc-sc.gc.ca/ewhsemt/pubs/contaminants/psl2lsp2/pm10/index-eng.php].
Horwell, C.J., and Baxter, P.J., 2006.The respiratory health hazards of volcanic ash: A review for volcanic risk mitigation: Bulletin of Volcanology, v. 69, p. 1-24.
Hough, R.L., 2007, Soil and human health: An epidemiological review: European Journal of Soil Science, v. 58, p. 12001212.
International Society of Exposure Analysis (ISEA), 2007, Use of in vitro bioaccessibility/relative bioavailability estimates for metals in regulatory settings: What is needed, in Proceedings of the International Society of Exposure Assessment, Durham, North Carolina October 4, 2007, [http://epa.gov/superfund/bioavailability/links.htm].
Jackson, S.A., 1992, Estimating radon potential from an aerial radiometric survey: Health Physics, The Radiation Protection Journal, v. 62, no. 5, p. 450-452.
Krewski, D., Lubin J.H., Zielinski, J. M., Alavanja, M., Catalan, V.S., Field, R.W., Klotz, J.B., Letourneau, E.G., Lynch, C.F., Lyon, J.I., Sandler, D.P., Schoenberg, J.B., Steck, D.J., Stolwijk, J.A., Weinberg, C., and Wilcox, H.B., 2005, Residential radon and risk of lung cancer: A combined analysis of 7 North American case-control studies: Epidemiology, v. 16, p. 137-145.
Leybourne, M.L., Peter, J.M., Johannesson, K.H., and Boyle, D.R., 2008, The Lake St. Martin bolide has a big impact on groundwater fluoride concentration: Geology, v. 36, p. 115-118.
Maddaloni, M., Lolacono, N., Manton, W., Blum, C., Drexler, J., and Graziano, J., 1998, Bioavailability of soilborne lead in adults by stable isotope dilution: Environmental Health Perspectives, v. 106 (Supplement 6), p. 1589-1594.
Manton, W.I., Angle, C.R., Stanek, K.L., Reese, Y.R., and Kuehnemann, T.J., 2000, Acquisition and retention of lead by young children: Environmental Research, v. 82, p. 60-80.
McLaughlin Centre, 2007a, Workshop Proceedings on Environmental Burden of Disease: McLaughlin Institute of Population Health and Health Canada, University of Ottawa, February 12, 2007, [www.mclaughlincentre.ca/events].
McLaughlin Centre, 2007b, Workshop Proceedings on the Use of Geoscience in Population Health Risk Assessment: McLaughlin Centre for Population Health Risk Assessment, Natural Resources Canada, Canadian Shield Research Institute, University of Ottawa, November 28, 2007, [www.mclaughlincentre.ca/events].
McLaughlin Centre, 2008, Workshop Proceedings on Health Risk Assessment of Essential Metals: McLaughlin Institute of Population Health, University of Ottawa, May 6-7, 2008, [www.mclaughlincentre.ca/events].
Merian, E., Anke, M., Ihnat, M, and Stoeppler, M., eds., 2004, Elements and their Compounds in the Environment – Occurrence, Analysis and Biological Relevance: Wiley-VCH, Weinheim, 3 volumes, 1774 p.
Metals in the Human Environment – Strategic Network (MITHE-SN), 2008, Canadian Network for Toxicology Centres, [http://www.mithesn.org/research/index.cfm].
Natural Resources Canada, 2007a, Reducing risks from metals: Environmental impacts of historical gold mines: Geologic Survey of Canada, ISBN: 978-0662-05035-3; ISSN: M34-4/3-2007, [http://gsc.nrcan.gc.ca/org/atlantic/gold_e.php].
Natural Resources Canada, 2007b, Radiation geophysics, radon: Geological Survey of Canada [http://gsc.nrcan.gc.ca/gamma/radon_e.php].
Natural Resources Canada, 2008, Environment and health program, North American soil geochemical landscapes project: Geologic Survey of Canada [http://ess.nrcan.gc.ca/ehesh/trinat/index_e.php].
Nicks, L.P., Calmback, L., and Merry, A.G., 2007, Ambient groundwater study in the Thames, Sydenham and region source protection area, in Summary of Field Work and Other Activities, 2007: Ontario Geological Survey, Open File Report 6213, p. 25-1 to 25-4.
Nolan, R.P., Langer, A.M., Ross, M., Wicks, F.J., and Martin, R.F., eds., 2001, Health Effects of Chrysotile Asbestos: Contribution of science to risk-management decisions: The Canadian Mineralogist, Special Publication 5, 304 p.
Olin, S.S., 1998, Between a rock and a hard place: Methods for setting dietary allowances and exposure limits for essential minerals: Journal of Nutrition, v. 128, p. 364S-367S.
Outridge, P.M., Scheuhammer, A.M., Fox, G.A., Braune, B.M., White, L.M., Gregorich, L.J., and Keddy, C., 1999, An assessment of the potential hazards of environmental selenium for Canadian water birds: Environmental Reviews, v. 7, p. 81-96.
Parsons, M.B. and Percival, J.B., eds., 2005, Mercury: sources, measurements, cycles, and effects: Mineralogical Association of Canada Short Course Series, v. 34, 298 p.
Rasmussen, P.E., 1993, The environmental significance of geological sources of mercury: A Precambrian Shield watershed study: Unpublished Ph.D. Thesis, Earth Sciences Department, University of Waterloo, 379 p.
Rasmussen, P.E., 1996, Trace metals in the environment: A geological perspective: Geological Survey of Canada, Bulletin 429, 26 p.
Rasmussen, P.E., Villard, D.J., Gardner, H.D., Fortescue, J.A.C., Schiff, S.L., and Shilts, W.W., 1998a, Mercury in lake sediments of the Precambrian Shield near Huntsville, Ontario, Canada: Environmental Geology, v. 33, p. 96-108.
Rasmussen, P.E., Friske, P.W.B., Azzaria, L.M., and Garrett, R.G., 1998b, Mercury in the Canadian environment: Current research challenges: Geoscience Canada, v. 25, p. 1-8.
Rasmussen, P.E., Subramanian, K.S., and Jessiman, B.J., 2001, A multi-element profile of house dust in relation to exterior dust and soils in the city of Ottawa, Canada: Science of the Total Environment, v. 267, p. 125-140.
Rasmussen, P.E., 2004a, Elements and their compounds in indoor environments, in Merian, E., Anke, M., Ihnat, M., and Stoeppler, M., eds., Elements and their Compounds in the Environment – Occurrence, Analysis and Biological Relevance, Wiley-VCH, Weinheim, v. 1, part 1, chapter 11, p. 215-234.
Rasmussen, P.E., 2004b, Can metal concentrations in indoor dust be predicted from soil geochemistry? Canadian Journal of Analytical Science and Spectroscopy, v. 49, p. 166-174.
Rasmussen, P.E., Edwards, G.C, Schroeder, W.H., Ausma, S., Steffen, A., Kemp, J., Hubble Fitzgerald, C., El Bilali, E., and Dias, G., 2005, Measurement of gaseous mercury flux in terrestrial environments, in Parsons, M.B. and Percival, J.B., eds., Mercury: Sources, Measurements, Cycles, and Effects: Mineralogical Association of Canada Short Course Series, v. 34, p 123-138.
Rasmussen, P.E., Dugandzic, R., Hassan, N., Murimboh, J., and Grégoire, C., 2006, Challenges in analysing airborne metal concentrations in residential environments: Canadian Journal of Analytical Science and Spectroscopy, v. 51, p. 1-8.
Rasmussen, P.E., Wheeler, A.J., Hassan, N.M., Filiatreault, A., and Lanouette, M., 2007a, Monitoring personal, indoor, and outdoor exposures to metals in airborne particulate matter: Risk of contamination during sampling, handling and analysis: Atmospheric Environment, v. 41, p. 5897-5907.
Rasmussen P.E., Finley, R., Petrovic, S., Jones-Otazo, H., Marro, L., Thuppal, V., Walker, M., Chénier, M., Lanouette, M., and Levesque, C., 2007b, Canadian house dust study, Part 1: Methodologies: Health Canada Science Forum, Marriott Hotel, Ottawa, Ontario, November 8-9, 2007, Poster and Abstract CHDS , ISBN: H1-9/232007E, p. 2.33.
Rasmussen, P.E., Beauchemin, S., Nugent, M., Dugandzic, R., Lanouette, M., and Chénier, M., 2008, Influence of matrix composition on bioaccessible copper, zinc and nickel in urban residential dust and soil: Human and Ecological Risk Assessment, v. 14, p. 351-371.
Reeder, R. J., Schoonen, M.A.A., and Lanzirotti, A., 2006, Metal speciation and its role in bioaccessibility and bioavailability: Reviews in Mineralogy and Geochemistry, v. 64, p. 59-113.
Reimann, C., and Garrett, R.G., 2005, Geochemical background – concept and reality: Science of the Total Environment, v. 350, p. 12–27.
Rencz, A.N., O’Driscoll, N.J., Hall, G.E.M., Peron, T., Telmer, K., and Burgess, N.M., 2003, Spatial variation and correlations of mercury levels in the terrestrial and aquatic components of a wetland dominated ecosystem: Kejimkujik Park, Nova Scotia, Canada: Water, Air, and Soil Pollution, v. 143, p. 271-288.
Richardson, G.M., 2002, Determining natural (background) arsenic soil concentrations in Yellowknife NWT, and deriving site-specific human healthbased remediation objectives for arsenic in the Yellowknife area: Final report, submitted by Risklogic Scientific Services Inc. to the Yellowknife Arsenic Soils Remediation Committee (YASRC), Yellowknife, April 2002.
Richardson, G.M., Bright, D.A., and Dodd, M., 2006, Do current standards of practice measure what is relevant to human exposure at contaminated sites? II: Oral bioaccessibility of contaminants in soil: Human and Ecological Risk Assessment, v. 12, p. 606-616.
Sahai, N., 2007, Medical mineralogy and geochemistry: An interfacial science: Elements, v. 3, p. 381-384.
Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U., and Smedley, P., eds., 2005, Essentials of Medical Geology, Impact of the Natural Environment on Public Health: Academic Press, 832 p.
Skinner H.C.W., and Berger, A.R., eds., 2003, Geology and Health: Closing the Gap: Oxford University Press, 192 p.
Stasiuk, M.K., Hickson, C.J., and Mulder, T., 2006, The vulnerability of Canada to volcanic hazards: Natural Hazards, v. 28, p. 563-589.
United States Environmental Protection Agency (US-EPA), 2007a, Guidance for evaluating the oral bioavailability of metals in soils for use in human health risk assessment, OSWER 9285.7-80.
United States Environmental Protection Agency (US-EPA), 2007b, Estimation of relative bioavailability of lead in soil and soil-like materials using in vivo and in vitro methods, OSWER 9285.777.
United States National Research Council (US-NRC), 2007, Earth materials and health: Research priorities for earth science and public health, Committee on Research Priorities for Earth Science and Public Health, National Research Council and Institute of Medicine of the National Academies, National Academy of Engineering, 500 Fifth Street, NW, Washington, DC 20001 ISBN-13: 978-0-309-10470-8, 188 p.
Van Oostdam, J., Donaldson, S., Feeley, N., Tremblay, N., Arnold, D., Ayotte, P., Bondy, G., Chan, L., Dewailly, E., Furgal, C., Gill, U., Kalhok, S., Kuhnlein, H., Loring, E., Muckle, G., Myles, E., Receveur, O., Stokker, Y., and Tracy, B., 2003, Toxic substances in the Arctic and associated effectshuman health: Minister of Indian Affairs and Northern Development, Northern Contaminants Program, Canadian Artic Assessment Report II, Human Health, p. 1-127.
World Health Organization (WHO), 2007, Environmental burden of disease: Country profile for Canada, Geneva, 2007, [http://www.who.int/quantifying_ehimpacts/countryprofiles/en/index.html].
Yukon Department of Environment, 2007, Protocol for the contaminated sites regulation under the Environment Act, Protocol No. 10: Determining background groundwater quality, Prepared pursuant to Part 6 – Administration, Section 21, Contaminated Sites Regulation, OIC 2002/171, [www.environmentyukon.gov.yk.ca/pdf/protocol10.pdf].
Zielinski, J., Carz, Z., Krewski, D., and Repacholi, M., 2006, World Health Organization’s International Radon Project: Journal of Toxicology and Environmental Health, Part A, v. 69, p. 759-769.