Brucite -
Industrial Mineral with a Future
George J. SimandlBritish Columbia Ministry of Energy, Mines and Petroleum Resources, PO 9333 STN PROV GOV’T, Victoria, BC, V8W 9N3 Canada
George.Simandl@gov.bc.ca
Suzanne Paradis
Geological Survey of Canada, Sidney, Natural Resources Canada, 9860 West Saanich Road, Sidney BC, V8L 4B2 Canada
Melanie Irvine
British Columbia Ministry of Energy, Mines and Petroleum Resources, PO 9333 STN PROV GOV’T, Victoria, BC, V8W 9N3 Canada
SUMMARY
Brucite, Mg(OH)2, is an uncommon mineral primarily known to mineral collectors, and to specialists studying contact metamorphic and ultramafic rocks. It is an environmentally friendly flame-retardant and is in commercial demand; it also represents a potential ore source for the metal, magnesium, which is itself in great demand. The present brucite market for flame-retardants is less than 50 000 tonnes annually, but it is increasing exponentially. Brucite has the advantage of not containing CO2; hence none is released during calcination, a positive feature in today’s society concerned with climate change. This review paper summarises the topic for scientists studying the thermodynamic properties of brucite, geologists studying its contact meta-morphic characteristics, exploration geologists and potential end-users. Given the demand for the mineral and metal, high-grade brucite deposits may become hot exploration targets within the next few years.SOMMAIRE
La brucite, Mg(OH)2, est un minéral plutôt rare connu surtout des collectionneurs de minéraux et des spécialistes du métamorphisme de contact et des roches ultramafiques. La brucite est un matériau ignifuge écologique qui est en demande commercialement; il représente aussi une source potentielle de magnésium métallique, pour lequel existe une forte demande. La demande actuelle de brucite comme matériau ignifuge est de moins de 50 000 tonnes annuellement, mais elle croît exponentiellement. La brucite a l'avantage de ne pas contenir de CO2; et donc, aucun CO2 n'est produit lors de sa calcination, caractéristique très appréciée en ces temps d'inquiétudes en regard des changements climatiques. Notre article de synthèse présente un résumé de la question à l'intention des scientifiques intéressés par les propriétés thermodynamique de la brucite, aux géologues intéressés par ses caractéristiques de minéral de métamorphisme de contact, ainsi qu'aux géologues en général et aux utilisateurs. Étant donné la demande de brucite comme minéral et comme source de métal, les bons gisements de brucite pourraient constituer des cibles d'exploration dans un avenir rapproché.INTRODUCTION
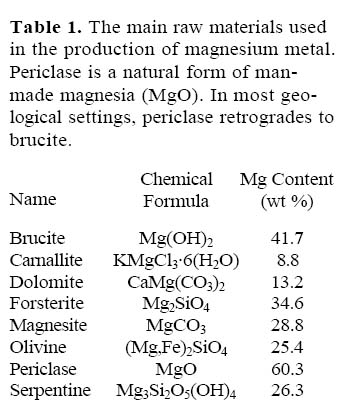
1 Brucite is a magnesium hydroxide Mg(OH)2. It has a higher magnesium content than any other raw material, commonly used or considered as ore (Table 1). Brucite forms soft, waxy to glassy, white, pale-green, grey or blue crystals, plate aggregates, rosettes, fibrous masses and fracture fillings. It is relatively soft (2.5 on the Mohs scale) and has a low density (2.38–2.40 g/cm3). It is soluble in hydrochloric acid but has no effervescence. Weathering transforms waxy, fresh brucite into a chalk-like material.
Table 1. The main raw materials used in the production of magnesium metal. Periclase is a natural form of man-made magnesia (MgO). In most geological settings, periclase retrogrades to brucite.
Display large image of Table 1
2 Brucite is widely distributed in ultramafic rocks (Khan et al. 1971; Hora 1998). It is also found in a variety of exotic settings such as kimberlites (Malkov 1974) and carbonatites (Lee et al. 2000). Most of the economic brucite deposits appear to be hosted by marbles affected by high-temperature, low-pressure metamorphism (mainly in pluton-associated, contact metamorphic aureoles). The fibrous variety of brucite, nemalite, is common in ultra-mafic rocks, where it coexists with chrysotile (Ross and Nolan 2003; Khan et al. 1971). Ultramafic-hosted deposits were considered as potential sources of brucite in the past (Khan et al. 1971) and are being considered again because brucite fibres from the Shaan Nan Asbestos Mine in Shaan Xi Province, China, were tested as a reinforcing material for concrete (Liu et al. 2004). The unfortunate association of brucite with asbestos in ultramafic settings, is the main reason why carbonate-hosted brucite deposits are the recommended and preferred exploration targets. The early negative studies on the inhalation effects of brucite dust are invalid because the supposedly pure brucite samples used in the experiments, contained up to 10% chrysotile (Davies et al. 1985). Liu et al. (2004) list a number of recent studies indicating that pure brucite is virtually harmless and this should allay some of the health concerns raised in earlier studies.
3 Examples of carbonate-hosted brucite deposits of economic significance are Cross Quarry near Wakefield, Québec, Canada (Jacob et al. 1991; Hébert and Paré 1990; Perreault 2003); Kuldur, eastern Russia (Anonymous 2005); Granåsen, Norway (Øvereng 2000); Gabbs magnesite– brucite deposit, Nye County, Nevada, USA (Schilling 1968) and Marble Canyon, Culberson County, Texas, USA (Newman and Hoffman 1996).
4 Other undeveloped or exhausted brucite deposits occur in China, Arizona, United Kingdom, Ireland, North Korea and Canada; however, fundamental, publicly available technical data are missing or not available.
5 World brucite reserves and resources are impossible to estimate with any reasonable accuracy because there are many inconsistencies in the reported numbers. Kramer (2001) estimates brucite reserves for Nevada, USA, at 3 million tonnes and for North Korea at 2 million tonnes; however, there are no indications of their grades. In Canada, under the 43-101CP Standards of Disclosure for mineral projects, such tonnage estimates cannot be referred to as reserves. Because the potential economic importance of brucite escaped the attention of most economic geologists, many carbonate-hosted occurrences worthy of geologic follow-up, such as those in British Columbia (Simandl et al. 2006), were never investigated in detail.
6 Brucite occurrences have recently been recognized in new geological settings, such as in accumulations on the sea floor (Kelley et al. 2001a, b; Früh-Green et al. 2003), and in the future brucite deposits belonging to this category may become of economic interest.
BRUCITE USES
7 The brucite market is relatively small, but it is growing rapidly. Brucite is classified either as a magnesium metal ore or as an industrial mineral. In both instances, it has to compete for its share of the market with other materials. According to the International Magnesium Association, the primary magnesium metal production for 2005 is estimated at 667 000 tonnes (Business Research Services Inc. 2006). Many common rock-forming minerals contain magnesium; however, brucite, carnallite, dolomite and magnesite are the main ore minerals (Coope 2004). Hydrated chlorites other than carnallite (such as bischofite), brines and seawater also represent important Mg resources. In addition, serpentine (including asbestos tailings) and olivine are possible raw materials. The extraction of magnesium from silicates is technologically feasible but economically challenging as was proven by the 2003 closure of Noranda’s Magnola plant located in Québec. Elemental magnesium (metal) and magnesium compounds are produced from bitterns, seawater, and well and lake brines (Coope 2004). Table 1 shows the magnesium content of the main raw materials used in magnesium metal production.
8 As there are no large, high-grade, developed brucite deposits in production, natural brucite is not currently used as raw material for magnesium metal extraction.
9 As an industrial mineral, brucite can be used in caustic and dead-burned magnesia production. It also has a variety of other industrial mineral applications such as a functional filler in plastic compounds, fire and smoke retardant (O’Driscoll 2005; Hornsby 2001; Simandl et al. 2001), electric wire insulation (Bisleri and Fondeur 2001) and carpet backing. There are no reliable statistics for the brucite market in any of these fields; however, the world market for flame retardant mineral fillers has been estimated at 500 000 tonnes; magnesium hydroxide accounts for approximately 10% (Rothon 2004). The magnesium hydroxide estimate includes natural and synthetic brucite. Natural brucite probably accounts for less than 25 000 tonnes in the field of flame-retardants.
10 Brucite is used also in waste-water treatment. For example, it has been proposed as one of the key minerals in Britannia Mine’s (BC) effluent treatment as a neutralizing reactant (Kus and Mavis 2001). Other uses include agricultural feed, a dietary magnesium supplement, odour control, and in specialty cement preparations as an additive to Portland cements (Godfrey 2000). There is promising laboratory-scale research into the use of brucite in stabilization of swelling clays (Xeidakis 1996). Brucite readily reacts with CO2 during mineral carbonation tests to form magnesium carbonates; however, brucite is not widely available (Chen et al. 2006).
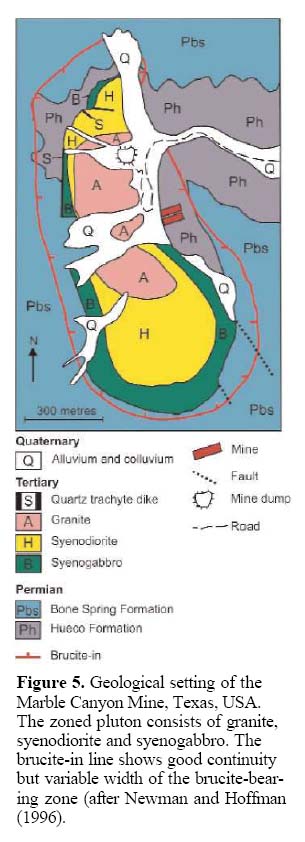
11 Depending on the intended industrial mineral use, natural brucite competes for its share of the market with synthetic brucite, commonly referred to in the manufacturing industry by its chemical formula, magnesium hydroxide, and with other minerals and compounds such as magnesite, dolomite, huntite, hydromagnesite, MgO, CaO, zeolites and others. The range of brucite’s price per tonne, in applications other than flame-retardants, is not available.
CONTACT METAMORPHIC BRUCITE DEPOSITS
12 Most of the brucite deposits of economic interest are associated with shallow-level igneous intrusions into MgO-bearing sedimentary or metasedimentary rocks. Examples of well documented, but not necessarily economic, brucite occurrences hosted by contact metamorphosed carbonates are described by Cartwright and Weaver (1993), Ferry (1996a, b, 2000), Ferry and Rumble (1997) and Müller et al. (2004). In these and most other well-documented cases, the brucite-bearing zone is located closest to the igneous intrusion.
13 Such studies are essential to understand the stability of brucite-bearing assemblages and ultimately the genesis of brucite deposits. The chemical composition of the host rock and that of the ambient fluids, appropriate pressure and temperature conditions, duration of the thermal event, lithological and structural controls, permeability (infiltration rather than diffusion), direction of fluid flow and fluid/rock ratio (open system) are some of the parameters that determine if brucite is formed and the shape and grade of the deposit.
Figure 1. Stability field of periclase and brucite in the CaO-MgO-CO2-H2O system at 1 kbar. The shapes of the curves indicate that the stabilities of periclase and brucite are strongly dependent on X (H2O) of the fluid (after von Trommsdorff and Schwander, 1969). Circled numbers refer to equations in the text. Abbreviations as follows: br-brucite, cc-calcite, dol-dolomite, p-periclase, m-magnesite.
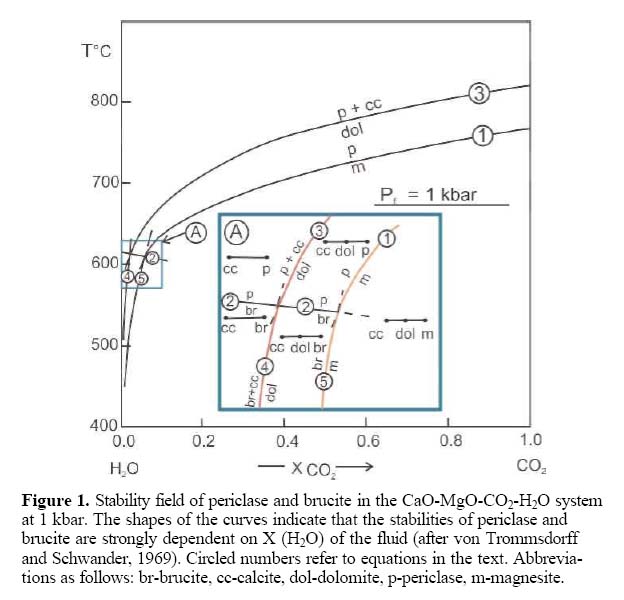
Display large image of Figure 1
14 Both dolomite and magnesite represent favourable host rocks for brucite formation (Fig. 1). The lower the mole fraction of carbon dioxide in the ambient fluid (X CO2), the lower the temperature required for brucite and periclase to be stable.
15 The highest grade, nearly monomineralic brucite deposits are found in aureoles where the protolith consists of magnesite with little or no calcite, clays, chert or other mineral impurities. This is believed to be the case for the Kuldur deposit (Shevelev 2004) and probably for the deposits at Gabbs. In these situations, periclase may have formed by dissociation of magnesite according to reaction (1): (1) Magnesite = Periclase (MgO) + CO2
Magnesite dissociation takes place during the prograde metamorphic (or metasomatic) phase. Later, periclase may rehydrate to form brucite during the retrograde phase by the hydration reaction (2): (2) Brucite = Periclase + H2O
Worldwide, sedimentary-hosted magnesite deposits such as those described by Simandl and Schultes (2004) represent an ideal protolith for the formation of high-grade brucite deposits, but they are uncommon. The settings where magnesite-rich rocks are affected by contact metamorphism or meta-somatism are, therefore, truly rare. Dolostones have lower magnesium content than magnesite-rich rocks so they are less likely to host nearly monomineralic brucite deposits. Dolomitic rocks are present over relatively wide areas in most geological settings. If the dolomite is pure, then Figure 1 appropriately represents the conditions of brucite formation. In such cases, periclase (MgO) is believed to have formed by a prograde, infiltration-driven reaction (3), referred to as dolomite dissociation: (3) Dolomite = Periclase + Calcite + CO2
In most contact metamorphic settings, periclase does not survive the retrograde metamorphism that follows a metamorphic climax and it rehydrates to form brucite according to reaction (2).
16 In settings where mole fraction of water (X H2O) is extremely high, brucite may form directly by reactions (4) and (5): (4) Dolomite + H2O = Brucite + Cal-cite + CO2
(5) Magnesite + H2O = Brucite + CO2
In most geological settings, almost pure carbonate consisting of dolomite ± magnesite cannot be expected. If the protolith is siliceous carbonate, then calc-silicate minerals will be part of the mineral paragenesis. Figure 2 illustrates the stability field of periclase, brucite and some of the other mineral assemblages that are expected at 1.5 kbar in silicious dolomite, a geological setting that can be approximated by a CaOMgO-SiO2-H2O-CO2 system.
17 Contact metamorphic aureoles that overprint siliceous dolomite-bearing carbonates are divided into two categories: those aureoles that, at the peak of high-grade metamorphism, contained periclase-forsterite-calcite and those that contained dolomiteforsterite-calcite (no periclase) assemblages.
18 If the prograde metamorphic peak assemblage is dolomite-forsteritecalcite, then brucite will form through reaction (6); however, during the retrograde stage forsterite may also react with calcite, H2O and CO2 to form tremolite and dolomite or serpentine and dolomite (Ferry 2000). (6) 34 Forsterite 20 Brucite + 51 H2O = Serpentine
As with most industrial minerals and metal ores, grade is one of the most important economic parameters. Therefore, exploration geologists should concentrate their efforts on geological settings with favourable bulk chemical composition where, X (CO2) may have approached zero (i.e. an open system rich in meteoric or magmatic fluids), temperature exceeded 600ºC and where permeability was
high during the peak conditions of contact metamorphism. Such settings coincide with the highest metamorphic grade of metamorphic aureoles (near intrusive-carbonate contacts and along nearby fault and fracture zones), where the hydrothermal fluid travels quickly without cooling down because of its reactions with the host rock.
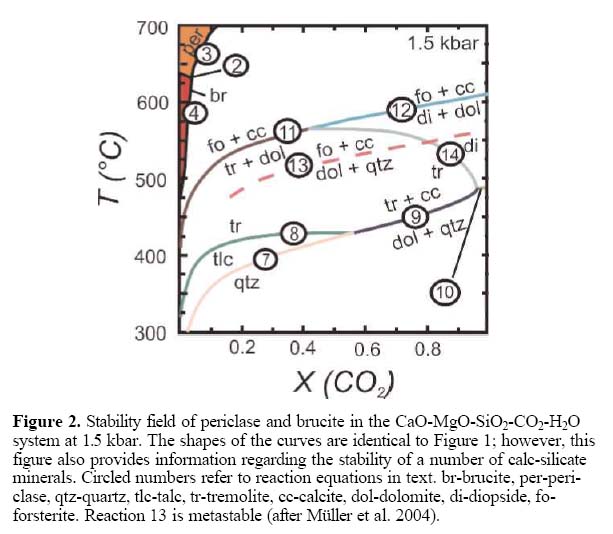
Display large image of Figure 2
19 It is essential that ambient fluids retain high X (H2O), low X (CO2) and the rocks remain calcite deficient for the optimum preservation of brucite; otherwise, destabilization of brucite will occur by reaction with cal-cite to form retrograde dolomite as shown in reaction (4).
20 Low silica activity in the ambient fluid also favours precipitation of brucite rather than the formation of serpentine or any other magnesium silicate. In many such cases, textural evidence suggests that periclase that formed during peak metamorphism was subsequently converted to brucite; however, this is probably not universal, and is not applicable to all brucite occurrences or deposits because brucite may also form by reactions 5 and 6.
21 Brucite may be destabilized in surface environments (regolith or out-crop) and converted to hydromagnesite and atinite (Schilling 1968).
22 The reactions explaining the sequence in which talc, tremolite, diop-side and forsterite appear in typical contact metamorphic environments, (Fig. 2), are listed below. (7) 3 Dolomite + 4 Quartz + 1 H2O
= 1 Talc + 3 Calcite + 3 CO2
(8) 5 Talc + 6 Calcite 4 Quartz =
3 Tremolite 6 CO2 +2 H2O
(9) 5 Dolomite + 8 Quartz 1 H2O
= 1 Tremolite + 3 Calcite + 7 CO2
(10) Dolomite + 2 Quartz = Diopside
+ CO
(11) Tremolite + 11 Dolomite
8 Forsterite + 13 Calcite H2O 9
CO
(12) 3 Dolomite + Diopside = 2
Forsterite + 4 Calcite + 2 CO2
(13) 0.21 Tremolite + 0.064 Calcite +
0.15 CO2 ! 0.105 Dolomite + 0.02
H2O + 0.171 SiO2gb (metastable)
(14) Tremolite + 3 Calcite = 4 Diopside
+ Dolomite + H2O + CO2
SELECTED EXAMPLES OF FAVOURABLE GEOLOGICAL SETTINGS FOR BRUCITE EXPLORATION
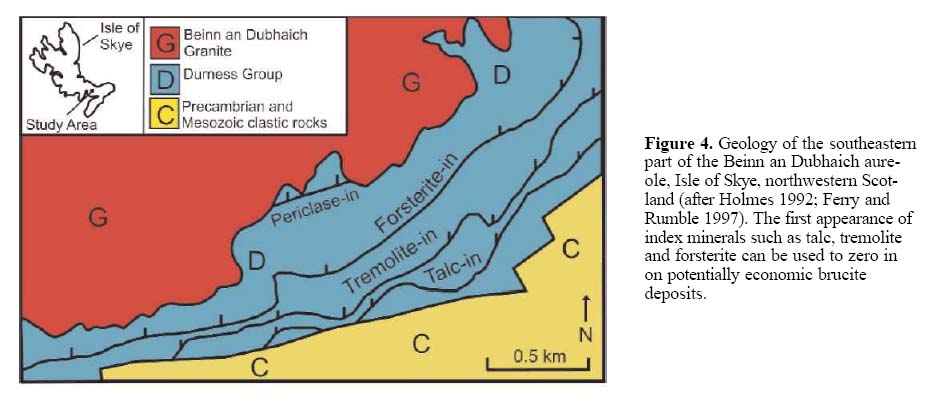
23 In most localities, where brucite occurs within impure dolomitic rocks (CaOMgO-SiO2-H2O-CO2 system) in a contact metamorphic environment, the reactions shown in Figure 2 apply. With increasing temperature and decreasing distance to the intrusive contact, there is a successive appearance of talc, tremolite, forsterite and periclase and/or brucite. This pattern has been found in several well-described localities. The Ubehebe Peak contact aureole, in California, USA (Fig. 3), is an excellent example showing the first appearance of index minerals tremolite, forsterite and periclase retrograded to brucite; at this locality talc was not reported. The absence of the diopside zone at this site was interpreted by Müller et al. (2004) as a result of fluid infiltration. A similar pattern is observed at the Beinn an Dubhaich aureole, northwest Scotland (Fig. 4; Holmes 1992; Ferry and Rumble 1997), but at this locality, talc was noted. Mineral zonations, together with the position of the heat source (igneous body) may be used in the exploration for brucite deposits to focus on favourable environments. Brucite-bearing zones depicted by Figures 3 and 4 are not known to contain economic brucite deposits; however, both show the either partial or complete metamorphic sequence of talc, tremolite, forsterite and periclase (commonly retrograded to brucite). Depending on the chemical composition of the protolith, hydrothermal fluids and pressure/temperature conditions, other minerals, such as wollastonite, may be present (Fig. 3).
24 Figure 5 shows the extent of the favourable brucite-bearing zone in the Marble Canyon area of Texas, which developed in dolomitic rocks that surrounded an elliptically shaped intrusive complex, consisting of horn-blende-bearing granite, syenodiorite and syenogabbro (Newman and Hoffman 1996). The brucite occurs within dolomitic rocks of the Hueco and Bone Springs formations (Fig. 5). The brucite-bearing halo surrounding the zoned intrusive body is up to 150 m wide. The protolith of the Hueco Formation is less siliceous than that of the Bone Springs Formation; consequently, it contains fewer calc-silicate minerals and has a better potential to host economic brucite deposits (Newman and Hoffman 1996). Applied Chemical Magnesias Corp. markets the brucite-bearing marble mined from this zone.
Figure 3. Mineral zoning within contact metamorphic aureole can be used to focus exploration efforts. In this example from Ubehebe Peak, Death Valley National Park, California, USA, the first appearance of tremolite (Tr), forsterite (Fo), and periclase (Per) indicates increasing temperature. Periclase formed in the hottest part of the contact zone, but was replaced by brucite. The appearance of wollastonite (Wo) may indicate local variation in the composition of the protolith. Pognip Limestone(Op), Eureka Quartzite (Oeq), Ely Springs Dolomite (Oes), Hidden Valley Dolomite (DShv), Lost Burro Formation (Dib), Perdido Formation (Mp), Tin Mountain Limestone (Mtm), alluvium (Qal), Paleozoic rocks (PAL) (from Roselle et al. 1999).
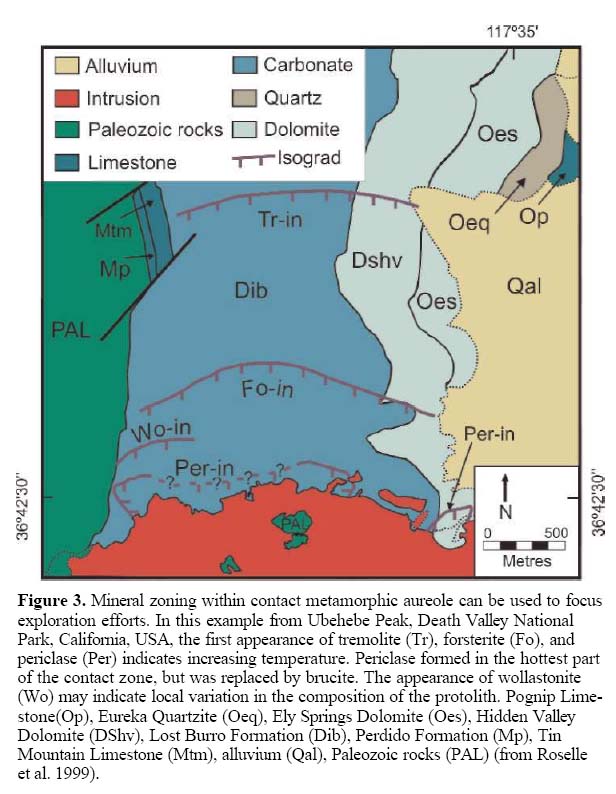
Display large image of Figure 3
SUMMARY AND GUIDELINES FOR MINERAL EXPLORATION AND DEVELOPMENT
25 Brucite currently has a relatively small market (Simandl et al. 2006), largely due to the rarity of high-grade deposits in comparison to other more common magnesium-bearing minerals, such as dolomite and magnesite. Large, high-grade brucite deposits could be a source of material for a variety of industrial mineral applications, such as manufacturing of caustic or dead-burned magnesia or for the production of magnesium metal. One of the most important advantages of brucite over magnesium-bearing carbonates is that it does not contain CO2 that would be released during calcination or processing. It is conceivable that the future use of brucite may involve CO2 credits. Brucite would be an excellent candidate for mineral sequestration if it were available on a large scale at a low to moderate price.
26 Although brucite is relatively widespread as an accessory mineral in a variety of rock types, Mg-rich carbonate horizons within contact metamorphic aureoles have the best exploration potential. Dolostone or magnesite-bearing rocks are the most favourable protoliths because they provide in situ sources of magnesia. In normal contact aureoles, exploration geologists should expect the following (or similar sequence): unaffected carbonate rock, first appearance of talc followed by first appearances of tremolite, forsterite with periclase and/or brucite directly in contact with the intrusive rock (Fig. 4). Also, exploration should not be limited to areas surrounding the pluton, as suggested by Figures 3, 4 and 5 because the roof pendants caught within the plutonic rocks offer ideal conditions for brucite formation as well.
27 There is also the possibility that the potential for formation of an economic brucite deposit increases when the marbles are in contact with silica-undersaturated rocks, such as syeno-gabbros (Fig. 5), or when the intrusive rock is a syenite, such as at the Stephen Cross Quarry in Québec.
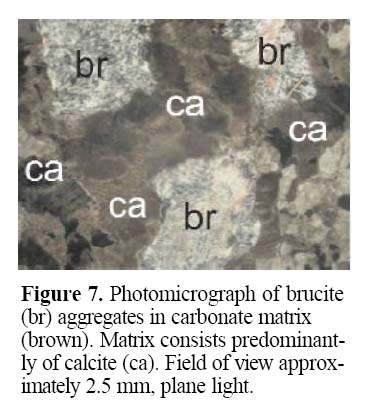
28 Due to its visible and near infrared spectra properties, remote sensing methods could identify brucite-rich rocks (Kozak and Duke 1999). The recessive nature of brucite deposits makes boulder tracing one of the most effective prospecting methods. Brucite-bearing carbonate boulders are mostly pale (white or grey), are characterized by mottled texture and have porous soft patches within the carbonate matrix (Figs. 6 and 7). Depending on the degree of weathering and ore grade, brucite can be altered to hydromagnesite or atinite for depths down to, or greater than, five metres. If brucite is formed within a dolomite rather than a magnesite protolith, the deposit is expected to have a lower grade and the alteration is not expected to be as deep.
CONCLUSION
29 Brucite is an industrial mineral with an excellent market-growth potential; it is currently used in a variety of niche markets such as fillers, flame retardant and in environment rehabilitation. The main reason why brucite is not used more widely is due to the rarity of large, high-grade deposits. Worldwide, there are several brucite occurrences similar to those reported in British Columbia (Simandl et al. 2006), where the extent of the brucite-bearing zone and brucite content is unknown. Such occurrences merit detailed follow-up exploration using guidelines highlighted here.
Figure 6. Brucite-bearing carbonate boulder characterized by mottled texture caused by white, porous, soft patches consisting of weathered brucite. The less weathered grey matrix consists of calcite. Figure 7. Photomicrograph of brucite (br) aggregates in carbonate matrix (brown). Matrix consists predominantly of calcite (ca). Field of view approximately 2.5 mm, plane light.ACKNOWLEDGEMENTS
Laura Simandl, from St. Margaret’s School in Victoria, collected the most spectacular brucite specimen at the Kennedy Lake Mine and also provided most of the project-related photographic documentation. Brian Grant and David Lefebure from the British Columbia Ministry of Energy, Mines and Petroleum Resources helped to improve earlier versions of this manuscript. Léopold Nadeau of the Geological Survey of Canada in Quebec City proof-read the final version.REFERENCES
Anonymous, 2005, The brucite of Kuldur deposit: Russian Mining Chemical Company. Online at: [http://www.brucite.ru/eng/]
Bisleri, C., and Fondeur, J.H., 2001, Fire-resistant and water-resistant halogen-free low-voltage cables, patent EP1128397: Online at: [http://v3.espacenet.com]
Business Research Services Inc., 2006, Years 2000-2005. Primary Magnesium Production: Online at: [http://www.intlmag.org/files/yend2005.pdf]
Cartwright, I., and Weaver, T.R., 1993, Fluid-rock interaction between syenites and marbles at Stephen Cross Quarry, Québec, Canada: petrological and stable isotope data: Contributions to Mineralogy and Petrology, v. 113, no. 4, p. 533-544.
Chen, Z.Y., O’Connor, W., and Gerde-mann, S.J., 2006, Chemistry of aqueous mineral carbonation for carbon sequestration and explanation of experimental results: Environmental Progress, v. 25, no. 2, p. 161-166.
Coope, B., 2004, Magnesium metal – sources, processes and markets, in Simandl, G.J., McMillan, W.J. and Robinson, N.D., eds., Proceedings of the 37th Forum on the Geology of Industrial Minerals, May 23-25, 2001, Victoria, BC, Canada: British Columbia Ministry of Energy and Mines, Paper 2004-2, p. 1-56.
Davis, J.M.G., Addison, J., Bolton, R.E., Donaldson, K., Jones, A.D., and Miller, B.D., 1985, Inhalation studies on the effects of tremolite and brucite dust in rats: Carcinogenesis, v. 6, no. 5, p. 667-674.
Ferry, J.M., 1996a, Three novel isograds in metamorphosed siliceous dolomites from the Ballachulish aureole: American Mineralogist, v. 81, p. 485-494.
Ferry, J.M., 1996b, Prograde and retrograde fluid flow during contact meta-morphism of siliceous carbonate rocks from the Ballachulish aureole, Scotland: Contribution to Mineralogy and Petrology, v. 124, p. 235-254.
Ferry, J.M., 2000, Patterns of mineral occurrences in metamorphic rocks: American Mineralogist, v. 85, p. 1573-1588.
Ferry, J.M. and Rumble, D., 1997, Formation and destruction of periclase by fluid flow in two contact aureoles: Contributions to Mineralogy and Petrology, v. 128, p. 313-334.
Früh-Green, G.L., Kelley, D.S., Bernasconi, S.M., Karson, J.A., Ludwig, K.A., Butterfield, D.A., Boschi, C., and Proskurowski, G., 2003, 30,000 years of hydrothermal activity at the Lost City Vent Field: Science, v. 301, p. 495-498.
Godfrey, P.J., 2000, Magnesium Cement Project Independent Appraisal: TecEco Pty Ltd., 23 p.
Hébert, Y., and Paré, C., 1990, Les ressources en minéraux de magnesium et leur utilisation au Québec: Ministère de l’Énergie et des Ressources du Québec, MB 90-31.
Holmes, M.B., 1992, Metamorphism and fluid infiltration of the calc-silicate aureole of the Beinn an Dubhaich granite, Skye: Journal of Petrology, v. 33, p.1261-1293.
Hora, Z.D., 1998, Ultramafic-hosted Chryssotile Asbestos, in Geological Fieldwork 1997: British Columbia Ministry of Employment and Investment, Paper 1998-1, p. 24K-1- 24K-4.
Hornsby, P.R., 2001, Fire retardant fillers for polymers: International Materials Reviews, v. 46, no.4, p. 199-210.
Jacob, H.-L., Cotnoir, D., Depatie, J., Goffaux, D., Dans, D., and Bergeron, M., 1991, Les Mineraux Industriel du Quebec cours intensif no.3: Association Professionelle des Geologues et Geophysiciens du Québec.
Kelley, D.S., Karson, J.A., Blackman, D.K., Früh-Green, G.L., Butterfield, D.A., Lilley, M.D., Olson, E.J., Schrenk, M.O., Roe, K.K., Lebon, G.T., and Rivizzigno, P, 2001a, An off axis hydrothermal vent field discovered near the Mid-Atlantic Ridge at 30°N: Nature, v. 412 (6843), p. 150–157.
Kelley, D. S., Karson, J., Blackman, D., Früh-Green, G., Butterfield, D., Lilley, M., Schrenk, M., Olson, E., Roe, K., and Lebon, J., Applegate, B., Bacher, N., Cann, J., Gee, J., Hanna, H., Hurst, S., John, J., Lyons, S., Morgan, J., Nooner, S., Ross, K., Sasagawa, G., and Schroeder, T., 2001b, Discovery of Lost City: An off-axis peridotite-hosted, hydrothermal field near 30°N on the Mid-Atlantic Ridge: Newsletter of the US RIDGE Initiative, v. 11, no. 2, p. 3-9.
Khan, S.A., Ali, K., and Alam, S. J., 1971, Brucite deposits of Hindubagh (West Pakistan): Pakistan Journal of Scientific and Industrial Research, v. 14, no. 6, p. 542-545.
Kozak, P.K., and Duke, E.F., 1999, Visible and near infrared spectra of contact metamorphosed calcareous rocks: implications for mapping metamorphic isograds by hyper spectral remote sensing methods: The Thirteenth International Conference on Applied Geological Remote Sensing, p. I-363-I-370.
Kramer, D.A., 2001, Magnesium, its Alloys and Compounds: US Geological Survey, Open-File Report 01-341, 29 p.
Kus, J., and Mavis, J., 2001, Britannia mitigation program – interim treatment, Britannia Mine Remediation Project, Corporate Land and Resource Governance: Ministry of Sustainable Resource Management, 2 p.
Lee, W.-J., Fanelli, M.F., Cava, N., and Wyllie, P.J., 2000, Calciocarbonatite and magnesiocarbonatite rocks and magmas represented in the system CaO-MgO-CO2-H2O at 0.2 GPa: Mineralogy and Petrology, v. 68, p. 225-256.
Liu, K., Cheng, H., and Zhow, J., 2004, Investigation of brucite-fiber-reinforced concrete: Cement and Concrete Research, v. 34, p.1981-1986.
Malkov, B.A., 1974, Brucite in kimberlite: Transactions (Doklady) of U.S.S.R Academy of Science, Earth Science Section, v. 215, p. 157-160.
Müller, T., Baumgartner, L.P., Foster, Jr., C.T., and Vennemann, T.W., 2004, Metastable prograde mineral reactions in contact aureoles: Geology, v. 32, no. 9, p. 821-824.
Newman, T.E., and Hoffman, G.K., 1996, Brucite deposit in Marble Canyon, Culberson County, Texas, in Austin, G.S., Hoffman, G.K., Barker, J.M., Zidek, J. and Gilson, N., eds., Proceed-st ings, 31 Forum on the Geology of Industrial Minerals: New Mexico Bureau of Mines and Natural Resources, Bulletin 154, p. 37-42.
O’Driscoll, M., 2005, Magnesia minors – brucite, huntite and hydromagnesite: Industrial Minerals, No. 453, Metal Bulletin Plc., p. 41-47.
Øvereng, O., 2000, Granåsen, a dolomitebrucite deposit with potential for industrial development: Norges Geologiske Undersøkelse, Bulletin 436, p. 75-84.
Perrault, S., 2003, The ups and downs of industrial mineral production in Québec: a response to changing markets: Newsletter of the Mineralogical Association of Canada, No. 71, p. 1-23.
Roselle, G.T., Baumgartner, L.P., and Valley, J.W., 1999, Stable isotope evidence of heterogeneous fluid infiltration at Ubehebe Peak, California: American Journal of Science, v. 299, p. 93-138.
Ross, M., and Nolan, R.P., 2003, History of asbestos discovery and use and asbestos-related disease in context with the occurrence of asbestos within ophiolite complexes: Geological Society of America, Special Paper 273, p. 447-470.
Rothon, R., 2004, European flame retardants: Industrial Minerals, No. 437, Metal Bulletin Plc., p. 35-39.
Schilling, J.H., 1968, The Gabbs magne-site-brucite deposit, Nye County, Nevada, in Ridge, J.D., ed., Ore Deposits of the United States, 1933-1967: Graton-Sales, American Institute of Mining, Metallurgical and Petroleum Engineers, New York, v. 2, p. 1608-1621.
Shevelev, A. I., 2004, Magnesite deposits of Russia: 32nd International Geological Congress, Florence, Italy, DWO 14-16.
Simandl, G.J., and Schultes, H., 2004, Classification of magnesite deposits with emphasis on the Mount Brussilof and Kunwara types, in Simandl, G.J., McMillan, W.J. and Robinson, N.D., eds., Proceedings of the 37th Forum on the Geology of Industrial Minerals, May 23-25, 2001, Victoria, BC, Canada: British Columbia Ministry of Energy and Mines, Paper 2004-2, p. 83-85.
Simandl, G. J., Simandl, J., and Debreceni, A., 2001, Hydromagnesite-magnesite resources: potential flame retardant material: British Columbia Ministry of Energy and Mines, Geological Fieldwork 2000, p. 327-336 Simandl, G. J., Paradis, S., Robinson, N. D,
Simandl, L., and Irvine, M.L., 2006, Brucite in British Columbia: British Columbia Ministry of Energy and Mines and Petroleum Resources, Geofile 2006-3.
von Trommsdorff, V., and Schwander, H., 1969, Brucitmarmore in den Bergelleralpen: Bulletin Suisse de Mineralogie et Petrographie, v. 49, no. 2, p. 333-340.
Xeidakis, G.S., 1996, Stabilization of swelling clays by Mg (OH)2. Changes in clay properties after addition of Mg-hydroxide: Engineering Geology, v. 44, p. 107-120.